Supercritical carbon dioxide-based sterilization of decellularized heart valves
- PMID: 28337488
- PMCID: PMC5358672
- DOI: 10.1016/j.jacbts.2016.08.009
Supercritical carbon dioxide-based sterilization of decellularized heart valves
Abstract
Objective: The goal of this research project encompasses finding the most efficient and effective method of decellularized tissue sterilization.
Background: Aortic tissue grafts have been utilized to repair damaged or diseased valves. Although, the tissues for grafting are collected aseptically, it does not eradicate the risk of contamination nor disease transfer. Thus, sterilization of grafts is mandatory. Several techniques have been applied to sterilize grafts; however, each technique shows drawbacks. In this study, we compared several sterilization techniques: supercritical carbon dioxide, electrolyzed water, gamma radiation, ethanol-peracetic acid, and hydrogen peroxide for impact on the sterility and mechanical integrity of porcine decellularized aortic valves.
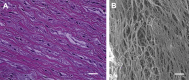
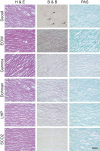
Methods: Valve sterility was characterized by histology, microbe culture, and electron microscopy. Uniaxial tensile testing was conducted on the valve cusps along their circumferential orientation to study these sterilization techniques on their integrity.
Results: Ethanol-peracetic acid and supercritical carbon dioxide treated valves were found to be sterile. The tensile strength of supercritical carbon dioxide treated valves (4.28 ± 0.22 MPa) was higher to those valves treated with electrolyzed water, gamma radiation, ethanol-peracetic acid and hydrogen peroxide (1.02 ± 0.15, 1.25 ± 0.25, 3.53 ± 0.41 and 0.37 ± 0.04 MPa, respectively).
Conclusions: Superior sterility and integrity were found in the decellularized porcine aortic valves with supercritical carbon dioxide sterilization. This sterilization technique may hold promise for other decellularized soft tissues.
Summary: Sterilization of grafts is essential. Supercritical carbon dioxide, electrolyzed water, gamma radiation, ethanol-peracetic acid, and hydrogen peroxide techniques were compared for impact on sterility and mechanical integrity of porcine decellularized aortic valves. Ethanol-peracetic acid and supercritical carbon dioxide treated valves were found to be sterile using histology, microbe culture and electron microscopy assays. The cusp tensile properties of supercritical carbon dioxide treated valves were higher compared to valves treated with other techniques. Superior sterility and integrity was found in the decellularized valves treated with supercritical carbon dioxide sterilization. This sterilization technique may hold promise for other decellularized soft tissues.
Keywords: Decontamination; decellularized; heart valve; tensile properties; tissue engineering.
Figures




Similar articles
-
Sterilization of Lung Matrices by Supercritical Carbon Dioxide.Tissue Eng Part C Methods. 2016 Mar;22(3):260-9. doi: 10.1089/ten.TEC.2015.0449. Epub 2016 Jan 29. Tissue Eng Part C Methods. 2016. PMID: 26697757 Free PMC article.
-
Low-Dose Gamma Irradiation of Decellularized Heart Valves Results in Tissue Injury In Vitro and In Vivo.Ann Thorac Surg. 2016 Feb;101(2):667-74. doi: 10.1016/j.athoracsur.2015.07.080. Epub 2015 Oct 9. Ann Thorac Surg. 2016. PMID: 26453425 Free PMC article.
-
A sterilization method for decellularized xenogeneic cardiovascular scaffolds.Acta Biomater. 2018 Feb;67:282-294. doi: 10.1016/j.actbio.2017.11.035. Epub 2017 Dec 2. Acta Biomater. 2018. PMID: 29183849
-
Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal.Bioact Mater. 2021 Feb 27;6(9):2927-2945. doi: 10.1016/j.bioactmat.2021.02.010. eCollection 2021 Sep. Bioact Mater. 2021. PMID: 33732964 Free PMC article. Review.
-
A new era for sterilization based on supercritical CO2 technology.J Biomed Mater Res B Appl Biomater. 2020 Feb;108(2):399-428. doi: 10.1002/jbm.b.34398. Epub 2019 May 27. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31132221 Review.
Cited by
-
Strategies for Development of Synthetic Heart Valve Tissue Engineering Scaffolds.Prog Mater Sci. 2023 Oct;139:101173. doi: 10.1016/j.pmatsci.2023.101173. Epub 2023 Jul 26. Prog Mater Sci. 2023. PMID: 37981978 Free PMC article.
-
Advances and Challenges of Tissue Vascular Scaffolds and Supercritical Carbon Dioxide Technology in Cardiovascular Diseases.Tissue Eng Regen Med. 2025 Apr;22(3):273-284. doi: 10.1007/s13770-025-00710-3. Epub 2025 Mar 3. Tissue Eng Regen Med. 2025. PMID: 40029563 Review.
-
Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges.Int J Mol Sci. 2022 Oct 27;23(21):13040. doi: 10.3390/ijms232113040. Int J Mol Sci. 2022. PMID: 36361824 Free PMC article. Review.
-
A Comparison of Sterilization Techniques for Production of Decellularized Intestine in Mice.Tissue Eng Part C Methods. 2020 Feb;26(2):67-79. doi: 10.1089/ten.TEC.2019.0219. Epub 2020 Jan 14. Tissue Eng Part C Methods. 2020. PMID: 31802699 Free PMC article.
-
Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications.J Tissue Eng Regen Med. 2018 Jul;12(7):1608-1620. doi: 10.1002/term.2686. Epub 2018 May 30. J Tissue Eng Regen Med. 2018. PMID: 29749108 Free PMC article.
References
-
- Galea G. The organization of tissue banking in Scotland. Scott Med J. 2012;57:225–231. - PubMed
-
- van Kats J.P., van Tricht C., van Dijk A. Microbiological examination of donated human cardiac tissue in heart valve banking. Eur J Cardiothorac Surg. 2010;37:163–169. - PubMed
-
- Uriarte J.J., Nonaka P.N., Campillo N. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. J Mech Behav Biomed Mater. 2014;40:168–177. - PubMed
-
- Rosario D.J., Reilly G.C., Ali Salah E., Glover M., Bullock A.J., Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. 2008;3:145–156. - PubMed
-
- Akkus O., Rimnac C.M. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927–934. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

