Effect of Sequential Treatment with Bisphosphonates After Teriparatide in Ovariectomized Rats: A Direct Comparison Between Risedronate and Alendronate
- PMID: 28337514
- PMCID: PMC5486924
- DOI: 10.1007/s00223-017-0263-6
Effect of Sequential Treatment with Bisphosphonates After Teriparatide in Ovariectomized Rats: A Direct Comparison Between Risedronate and Alendronate
Abstract
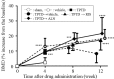
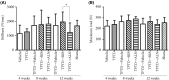
Teriparatide (TPTD), a recombinant human parathyroid hormone N-terminal fragment (1-34), is a widely used bone anabolic drug for osteoporosis. Sequential treatment with antiresorptives such as bisphosphonates after TPTD discontinuation is generally recommended. However, relative effects of bisphosphonates have not been determined. In the present study, we directly compared effects of risedronate (RIS) and alendronate (ALN) on bone mineral density (BMD), bone turnover, structural property and strength in ovariectomized (OVX) rats, when administered after TPTD. Female Sprague Dawley rats were divided into one sham-operated and eight ovariectomized groups. TPTD, RIS, and ALN were given subcutaneously twice per week for 4 or 8 weeks after 4 week treatment with TPTD. TPTD significantly increased BMD (+9.6%) in OVX rats after 4 weeks of treatment. 8 weeks after TPTD withdrawal, vehicle-treated group showed a blunted BMD increase of +8.4% from the baseline. In contrast, 8 weeks of treatment with RIS and ALN significantly increased BMD to 17.4 and 21.8%, respectively. While ALN caused a consistently larger increase in BMD, sequential treatment with RIS resulted in lower Tb.Sp compared to ALN in the fourth lumbar vertebra as well as in greater stiffness in compression test. In conclusion, the present study demonstrated that sequential therapy with ALN and RIS after TPTD both improved bone mass and structure. Our results further suggest that RIS may have a greater effect on improving bone quality and stiffness than ALN despite less prominent effect on BMD. Further studies are necessary to determine clinical relevance of these findings to fracture rate.
Keywords: Alendronate; Bone architecture; Bone quality; Risedronate; Teriparatide.
Conflict of interest statement
Conflict of interest
Authors Tetsuo Yano and Mei Yamada are employees of EA Pharma Co., Ltd. and Ajinomoto Co., Inc, respectively. Daisuke Inoue served as a consultant for EA Pharma.
Human and animal rights and informed consent
This article does not contains any studies with human participants performed by any of the authors. All procedures performed in the study were approved by the Animal Care and Use Committee of EA Pharma Co. Ltd. and PharmaLegacy Laboratories IACUC.
Figures


References
-
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. - DOI - PubMed
-
- Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, Lindsay R, Mitlak BH. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510–516. doi: 10.1007/s00198-004-1713-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

