BACE2 suppression promotes β-cell survival and function in a model of type 2 diabetes induced by human islet amyloid polypeptide overexpression
- PMID: 28337562
- PMCID: PMC11107557
- DOI: 10.1007/s00018-017-2505-1
BACE2 suppression promotes β-cell survival and function in a model of type 2 diabetes induced by human islet amyloid polypeptide overexpression
Abstract
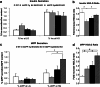
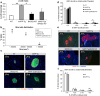
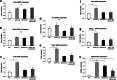
BACE2 (β-site APP-cleaving enzyme 2) is a protease expressed in the brain, but also in the pancreas, where it seems to play a physiological role. Amyloidogenic diseases, including Alzheimer's disease and type 2 diabetes (T2D), share the accumulation of abnormally folded and insoluble proteins that interfere with cell function. In T2D, islet amyloid polypeptide (IAPP) deposits have been shown to be a pathogenic key feature of the disease. The aim of the present study was to investigate the effect of BACE2 modulation on β-cell alterations in a mouse model of T2D induced by IAPP overexpression. Heterozygous mice carrying the human transcript of IAPP (hIAPP-Tg) were used as a model to study the deleterious effects of IAPP upon β-cell function. These animals showed glucose intolerance and impaired insulin secretion. When crossed with BACE2-deficient mice, the animals presented a significant improvement in glucose tolerance accompanied with an enhanced insulin secretion, as compared to hIAPP-Tg mice. BACE2 deficiency also partially reverted gene expression changes observed in islets from hIAPP-Tg mice, including a set of genes related to inflammation. Moreover, homozygous hIAPP mice presented a severe hyperglycemia and a high lethality rate from 8 weeks onwards due to a massive destruction of β-cell mass. This process was significantly reduced when crossed with the BACE2-KO model, improving the survival rate of the animals. Altogether, the absence of BACE2 ameliorates glucose tolerance defects induced by IAPP overexpression in the β-cell and promotes β-cell survival. Thus, targeting BACE2 may represent a promising therapeutic strategy to improve β-cell function in T2D.
Keywords: BACE activity; Glucose tolerance; Islet inflammation; Proliferation; Survival; Type 2 diabetes.
Figures



Similar articles
-
Inhibition of BACE2 counteracts hIAPP-induced insulin secretory defects in pancreatic β-cells.FASEB J. 2015 Jan;29(1):95-104. doi: 10.1096/fj.14-255489. Epub 2014 Oct 23. FASEB J. 2015. PMID: 25342134
-
Alpha1-antitrypsin ameliorates islet amyloid-induced glucose intolerance and β-cell dysfunction.Mol Metab. 2020 Jul;37:100984. doi: 10.1016/j.molmet.2020.100984. Epub 2020 Mar 27. Mol Metab. 2020. PMID: 32229246 Free PMC article.
-
Identification of Human Islet Amyloid Polypeptide as a BACE2 Substrate.PLoS One. 2016 Feb 3;11(2):e0147254. doi: 10.1371/journal.pone.0147254. eCollection 2016. PLoS One. 2016. PMID: 26840340 Free PMC article.
-
Human IAPP amyloidogenic properties and pancreatic β-cell death.Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review.
-
Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes.ILAR J. 2006;47(3):225-33. doi: 10.1093/ilar.47.3.225. ILAR J. 2006. PMID: 16804197 Review.
Cited by
-
Genetic discovery and risk characterization in type 2 diabetes across diverse populations.HGG Adv. 2021 Apr 8;2(2):100029. doi: 10.1016/j.xhgg.2021.100029. Epub 2021 Mar 9. HGG Adv. 2021. PMID: 34604815 Free PMC article.
-
Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12158-12163. doi: 10.1073/pnas.1808855115. Epub 2018 Nov 14. Proc Natl Acad Sci U S A. 2018. PMID: 30429322 Free PMC article.
-
Highly Selective and Potent Human β-Secretase 2 (BACE2) Inhibitors against Type 2 Diabetes: Design, Synthesis, X-ray Structure and Structure-Activity Relationship Studies.ChemMedChem. 2019 Mar 5;14(5):545-560. doi: 10.1002/cmdc.201800725. Epub 2019 Feb 5. ChemMedChem. 2019. PMID: 30637955 Free PMC article.
-
Hepatitis B X-interacting protein promotes the formation of the insulin gene-transcribing protein complex Pdx-1/Neurod1 in animal pancreatic β-cells.J Biol Chem. 2018 Feb 9;293(6):2053-2065. doi: 10.1074/jbc.M117.809582. Epub 2017 Dec 19. J Biol Chem. 2018. PMID: 29259128 Free PMC article.
-
Responsiveness to endurance training can be partly explained by the number of favorable single nucleotide polymorphisms an individual possesses.PLoS One. 2023 Jul 20;18(7):e0288996. doi: 10.1371/journal.pone.0288996. eCollection 2023. PLoS One. 2023. PMID: 37471354 Free PMC article. Clinical Trial.
References
-
- Saunders A, Kim T-W, Tanzi R. BACE maps to chromosome 11 and a BACE homolog, BACE2, reside in the obligate Down Syndrome region of chromosome 21. Science. 1999;286:1255a. doi: 10.1126/science.286.5443.1255a. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

