Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth
- PMID: 28337745
- PMCID: PMC5538190
- DOI: 10.1113/JP273330
Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth
Abstract
The placenta is the main determinant of fetal growth and development in utero. It supplies all the nutrients and oxygen required for fetal growth and secretes hormones that facilitate maternal allocation of nutrients to the fetus. Furthermore, the placenta responds to nutritional and metabolic signals in the mother by altering its structural and functional phenotype, which can lead to changes in maternal resource allocation to the fetus. The molecular mechanisms by which the placenta senses and responds to environmental cues are poorly understood. This review discusses the role of the insulin-like growth factors (IGFs) in controlling placental resource allocation to fetal growth, particularly in response to adverse gestational environments. In particular, it assesses the impact of the IGFs and their signalling machinery on placental morphogenesis, substrate transport and hormone secretion, primarily in the laboratory species, although it draws on data from human and other species where relevant. It also considers the role of the IGFs as environmental signals in linking resource availability to fetal growth through changes in the morphological and functional phenotype of the placenta. As altered fetal growth is associated with increased perinatal morbidity and mortality and a greater risk of developing adult-onset diseases in later life, understanding the role of IGFs during pregnancy in regulating placental resource allocation to fetal growth is important for identifying the mechanisms underlying the developmental programming of offspring phenotype by suboptimal intrauterine growth.
Keywords: IGF; fetus; nutrient transport; placenta; pregnancy; resource allocation; signalling.
© 2017 University of Cambridge. The Journal of Physiology © 2017 The Physiological Society.
Figures
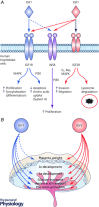
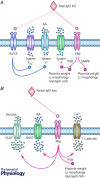
Comment in
-
Stress during pregnancy and its life-long consequences for the infant.J Physiol. 2017 Aug 1;595(15):5055-5056. doi: 10.1113/JP274444. J Physiol. 2017. PMID: 28762512 Free PMC article. No abstract available.
References
-
- Abu‐Amero SN, Ali Z, Bennett P, Vaughan JI & Moore GE (1998). Expression of the insulin‐like growth factors and their receptors in term placentas: a comparison between normal and IUGR births. Mol Reprod Dev 49, 229–235. - PubMed
-
- Ain R, Canham LN & Soares MJ (2005). Dexamethasone‐induced intrauterine growth restriction impacts the placental prolactin family, insulin‐like growth factor‐II and the Akt signaling pathway. J Endocrinol 185, 253–263. - PubMed
-
- Akram SK, Akram M, Bhutta ZA & Soder O (2008). Human placental IGF‐I and IGF‐II expression: correlating maternal and infant anthropometric variables and micronutrients at birth in the Pakistani population. Acta Paediatrica 97, 1443–1448. - PubMed
-
- Anderson AH, Fennessey PV, Meschia G, Wilkening RB & Battaglia FC (1997). Placental transport of threonine and its utilization in the normal and growth‐restricted fetus. Am J Physiol Endocrinol Metab 272, E892–E900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

