Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox
- PMID: 28337966
- PMCID: PMC11687472
- DOI: 10.1128/microbiolspec.TBTB2-0003-2015
Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox
Abstract
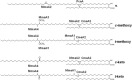
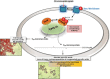
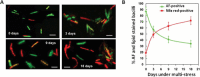
Acid-fast (AF) staining, also known as Ziehl-Neelsen stain microscopic detection, developed over a century ago, is even today the most widely used diagnostic method for tuberculosis. Herein we present a short historical review of the evolution of AF staining methods and discuss Koch's paradox, in which non-AF tubercle bacilli can be detected in tuberculosis patients or in experimentally infected animals. The conversion of Mycobacterium tuberculosis from an actively growing, AF-positive form to a nonreplicating, AF-negative form during the course of infection is now well documented. The mechanisms of loss of acid-fastness are not fully understood but involve important metabolic processes, such as the accumulation of triacylglycerol-containing intracellular inclusions and changes in the composition and spatial architecture of the cell wall. Although the precise component(s) responsible for the AF staining method remains largely unknown, analysis of a series of genetically defined M. tuberculosis mutants, which are attenuated in mice, pointed to the primary role of mycolic acids and other cell wall-associated (glyco)lipids as molecular markers responsible for the AF property of mycobacteria. Further studies are now required to better describe the cell wall reorganization that occurs during dormancy and to develop new staining procedures that are not affected by such cell wall alterations and that are capable of detecting AF-negative cells.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

