Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers
- PMID: 28338051
- PMCID: PMC5364539
- DOI: 10.1038/srep45170
Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers
Erratum in
-
Corrigendum: Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers.Sci Rep. 2018 May 22;8:46989. doi: 10.1038/srep46989. Sci Rep. 2018. PMID: 29786692 Free PMC article.
Abstract
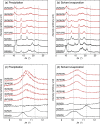
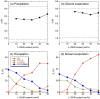
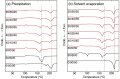
D-configured poly(D-lactic acid) (D-PLA) and poly(D-2-hydroxy-3-methylbutanoic acid) (D-P2H3MB) crystallized separately into their homo-crystallites when crystallized by precipitation or solvent evaporation, whereas incorporation of L-configured poly(L-2-hydroxybutanoic acid) (L-P2HB) in D-configured D-PLA and D-P2H3MB induced co-crystallization or ternary stereocomplex formation between D-configured D-PLA and D-P2H3MB and L-configured L-P2HB. However, incorporation of D-configured poly(D-2-hydroxybutanoic acid) (D-P2HB) in D-configured D-PLA and D-P2H3MB did not cause co-crystallization between D-configured D-PLA and D-P2H3MB and D-configured D-P2HB but separate crystallization of each polymer occurred. These findings strongly suggest that an optically active polymer (L-configured or D-configured polymer) like unsubstituted or substituted optically active poly(lactic acid)s can act as "a configurational or helical molecular glue" for two oppositely configured optically active polymers (two D-configured polymers or two L-configured polymers) to allow their co-crystallization. The increased degree of freedom in polymer combination is expected to assist to pave the way for designing polymeric composites having a wide variety of physical properties, biodegradation rate and behavior in the case of biodegradable polymers.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Synthesis and stereocomplex formation of enantiomeric alternating copolymers with two types of chiral centers, poly(lactic acid-alt-2-hydroxybutanoic acid)s.RSC Adv. 2020 Oct 23;10(64):39000-39007. doi: 10.1039/d0ra08351h. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518423 Free PMC article.
-
Ternary Stereocomplex Formation of One l-Configured and Two d-Configured Optically Active Polyesters, Poly(l-2-hydroxybutanoic acid), Poly(d-2-hydroxybutanoic acid), and Poly(d-lactic acid).ACS Macro Lett. 2012 Jun 19;1(6):687-691. doi: 10.1021/mz300155f. Epub 2012 May 17. ACS Macro Lett. 2012. PMID: 35607088
-
Corrigendum: Configurational Molecular Glue: One Optically Active Polymer Attracts Two Oppositely Configured Optically Active Polymers.Sci Rep. 2018 May 22;8:46989. doi: 10.1038/srep46989. Sci Rep. 2018. PMID: 29786692 Free PMC article.
-
Poly(lactic acid) stereocomplexes: A decade of progress.Adv Drug Deliv Rev. 2016 Dec 15;107:97-135. doi: 10.1016/j.addr.2016.04.017. Epub 2016 Apr 25. Adv Drug Deliv Rev. 2016. PMID: 27125192 Review.
-
Poly (lactic acid) blends: Processing, properties and applications.Int J Biol Macromol. 2019 Mar 15;125:307-360. doi: 10.1016/j.ijbiomac.2018.12.002. Epub 2018 Dec 7. Int J Biol Macromol. 2019. PMID: 30528997 Review.
Cited by
-
Synthesis and stereocomplex formation of enantiomeric alternating copolymers with two types of chiral centers, poly(lactic acid-alt-2-hydroxybutanoic acid)s.RSC Adv. 2020 Oct 23;10(64):39000-39007. doi: 10.1039/d0ra08351h. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518423 Free PMC article.
-
Effects of Amphiphilic Chitosan on Stereocomplexation and Properties of Poly(lactic acid) Nano-biocomposite.Sci Rep. 2018 Mar 12;8(1):4351. doi: 10.1038/s41598-018-22281-1. Sci Rep. 2018. PMID: 29531341 Free PMC article.
-
Novel Self-Forming Nanosized DDS Particles for BNCT: Utilizing A Hydrophobic Boron Cluster and Its Molecular Glue Effect.Cells. 2022 Oct 21;11(20):3307. doi: 10.3390/cells11203307. Cells. 2022. PMID: 36291173 Free PMC article.
References
-
- Vert M., Feijen J., Albertsson A.-C., Scott G., Chiellini E., Eds, Biodegradable polymers and plastics, Cambridge: Royal Society of Chemistry, 1992.
-
- Mobley D. P., Ed., Plastics from microbes, New York: Hanser Publishers, 1994.
-
- Vert M., Schwarch G. & Coudane J. Present and Future of PLA Polymers. J. Macromol. Sci. Pure Appl. Chem., A32, 787–96 (1995).
-
- Domb A. J., Kost J., Wieseman D. M., Eds, Handbook of biodegradable polymers (Drug Targeting and Delivery, vol. 7), Amsterdam (The Netherlands): Harwood Academic Publishers, 1997.
-
- Kaplan D. L., Ed., Biopolymers from renewable resources, Springer: Berlin (Germany) 1998.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

