The Effect Of microbial Mats In The Decay Of Anurans With Implications For Understanding Taphonomic Processes In The Fossil Record
- PMID: 28338095
- PMCID: PMC5364532
- DOI: 10.1038/srep45160
The Effect Of microbial Mats In The Decay Of Anurans With Implications For Understanding Taphonomic Processes In The Fossil Record
Abstract
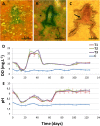
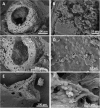
The pattern and sequence of the decomposition of the Pipidae African dwarf frog (Hymenochirus boettgeri) is tracked in an experiment with microbial mats in order to explore soft tissue preservation over three years. Frog decay in microbial mats is preceded by rapid entombment (25-30 days) and mediated by the formation of a sarcophagus, which is built by a complex microbial community. The frog carcasses maintained a variety of soft tissues for years. Labile organic structures show greater durability within the mat, cells maintain their general shape (bone marrow cells and adipocytes), and muscles and connective tissues (adipose and fibrous tendons) exhibit their original organic structures. In addition, other soft tissues are promptly mineralized (day 540) in a Ca-rich carbonate phase (encephalic tectum) or enriched in sulphur residues (integumentary system). The result is coherent with a bias in soft-tissue preservation, as some tissues are more likely to be conserved than others. The outcomes support observations of exceptionally preserved fossil anurans (adults and tadpoles). Decomposition in mats shows singular conditions of pH and dissolved oxygen. Mineralization processes could be more diverse than in simple heterotrophic biofilms, opening new taphonomic processes that have yet to be explored.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Gill-King H. In Forensic taphonomy: the postmortem fate of human remains (eds Sorg M. H. & Haglund W. D.) 93–108 (CRC Press, 1996).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

