The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3
- PMID: 28338101
- PMCID: PMC5364405
- DOI: 10.1038/srep45266
The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3
Abstract
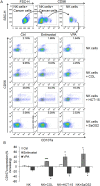
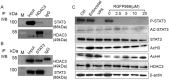
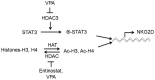
NKG2D is a major activating receptor of NK cells and plays a critical role in tumor immunosurveillance. NKG2D expression in NK cells is inhibited by the histone deacetylase (HDAC) inhibitor valproic acid (VPA) and enhanced by the narrow-spectrum HDAC inhibitor entinostat. We previously demonstrated that entinostat enhanced NKG2D transcription by increasing acetylation of Histones H3 and H4. However, the mechanism by which VPA reduces NKG2D expression in NK cells is not known. We have also shown that NKG2D transcription is regulated by STAT3 phosphorylation. In this study, we investigated regulation of NKG2D expression in NK cells by VPA and entinostat by assessing protein expression, phosphorylation, and interaction of HDACs and STAT3. We find that VPA selectively inhibits STAT3 tyrosine705 phosphorylation, but entinostat does not. STAT3 complexes with HDAC3, and HDAC3 inhibition represses STAT3 phosphorylation and therefore NKG2D expression. NK cells from STAT3 wild-type mice downregulate NKG2D in response to VPA, but not NK cells from STAT3 knockout mice. These results show that VPA is a potent inhibitor of STAT3 phosphorylation and demonstrate that histone acetylation and STAT3 tyrosine705 phosphorylation cooperate in regulating NKG2D expression in NK cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Lanier L. L. NK cell recognition. Annu Rev Immunol 23, 225–274 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

