Intracellular delivery of proteins by nanocarriers
- PMID: 28338410
- PMCID: PMC5829369
- DOI: 10.2217/nnm-2016-0393
Intracellular delivery of proteins by nanocarriers
Abstract
Intracellular delivery of proteins is potentially a game-changing approach for therapeutics. However, for most applications, the protein needs to access the cytosol to be effective. A wide variety of strategies have been developed for protein delivery, however access of delivered protein to the cytosol without acute cytotoxicity remains a critical issue. In this review we discuss recent trends in protein delivery using nanocarriers, focusing on the ability of these strategies to deliver protein into the cytosol.
Keywords: cytosolic delivery; nanoparticle-stabilized nanocapsules; nuclear targeting; protein; punctate fluorescence.
Conflict of interest statement
This work is supported by (GM077173) and the NSF (CHE-1506725). F Scaletti gratefully acknowledges FIRC (Italian Foundation for Cancer Research, Project Code: 18116) for the financial support. R Yu acknowledges the Young Faculty Study Abroad Program of Northwest A&F University for financial support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

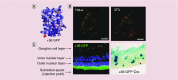

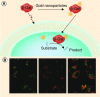
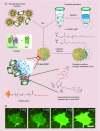
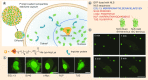

Similar articles
-
Direct delivery of functional proteins and enzymes to the cytosol using nanoparticle-stabilized nanocapsules.ACS Nano. 2013 Aug 27;7(8):6667-6673. doi: 10.1021/nn402753y. Epub 2013 Jul 8. ACS Nano. 2013. PMID: 23815280 Free PMC article.
-
Redox-responsive nanocapsules for intracellular protein delivery.Biomaterials. 2011 Aug;32(22):5223-30. doi: 10.1016/j.biomaterials.2011.03.060. Epub 2011 Apr 22. Biomaterials. 2011. PMID: 21514660
-
Protein corona change the drug release profile of nanocarriers: the "overlooked" factor at the nanobio interface.Colloids Surf B Biointerfaces. 2014 Nov 1;123:143-9. doi: 10.1016/j.colsurfb.2014.09.009. Epub 2014 Sep 16. Colloids Surf B Biointerfaces. 2014. PMID: 25262409
-
Nanostructures for protein drug delivery.Biomater Sci. 2016 Feb;4(2):205-18. doi: 10.1039/c5bm00360a. Biomater Sci. 2016. PMID: 26580477 Review.
-
Rational Design of Nanocarriers for Intracellular Protein Delivery.Adv Mater. 2019 Nov;31(46):e1902791. doi: 10.1002/adma.201902791. Epub 2019 Sep 8. Adv Mater. 2019. PMID: 31496027 Review.
Cited by
-
A small-molecule carrier for the intracellular delivery of a membrane-impermeable protein with retained bioactivity.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2407515121. doi: 10.1073/pnas.2407515121. Epub 2024 Oct 22. Proc Natl Acad Sci U S A. 2024. PMID: 39436658 Free PMC article.
-
A stimulus-responsive hexahedron DNA framework facilitates targeted and direct delivery of native anticancer proteins into cancer cells.Chem Sci. 2022 Aug 13;13(37):11132-11139. doi: 10.1039/d2sc02858a. eCollection 2022 Sep 28. Chem Sci. 2022. PMID: 36320481 Free PMC article.
-
Nanoencapsulation of Hirudo medicinalis proteins in liposomes as a nanocarrier for inhibiting angiogenesis through targeting VEGFA in the Breast cancer cell line (MCF-7).Bioimpacts. 2022;12(2):115-126. doi: 10.34172/bi.2021.39. Epub 2021 Aug 9. Bioimpacts. 2022. PMID: 35411300 Free PMC article.
-
Polymer Nanocontainers for Intracellular Delivery.Angew Chem Int Ed Engl. 2020 Feb 17;59(8):2962-2972. doi: 10.1002/anie.201907484. Epub 2019 Oct 31. Angew Chem Int Ed Engl. 2020. PMID: 31364243 Free PMC article. Review.
-
Codelivery of a cytotoxin and photosensitiser via a liposomal nanocarrier: a novel strategy for light-triggered cytosolic release.Nanoscale. 2018 Nov 8;10(43):20366-20376. doi: 10.1039/c8nr04048f. Nanoscale. 2018. PMID: 30376028 Free PMC article.
References
-
- Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 2008;7(1):21–39. - PubMed
-
- Walsh G. Biopharmaceutical benchmarks 2010. Nat. Biotech. 2010;28(9):917–924. - PubMed
-
- Gu Z, Biswas A, Zhao M, Tang Y. Tailoring nanocarriers for intracellular protein delivery. Chem. Soc. Rev. 2011;40(7):3638–3655. - PubMed
-
Comprehensive review on intracellular protein delivery. This is a tutorial review that talks about methodologies developed for intracellular delivery of proteins using nanocarriers.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
