Role and Function of A2A and A₃ Adenosine Receptors in Patients with Ankylosing Spondylitis, Psoriatic Arthritis and Rheumatoid Arthritis
- PMID: 28338619
- PMCID: PMC5412283
- DOI: 10.3390/ijms18040697
Role and Function of A2A and A₃ Adenosine Receptors in Patients with Ankylosing Spondylitis, Psoriatic Arthritis and Rheumatoid Arthritis
Abstract
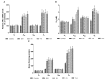
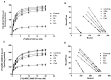
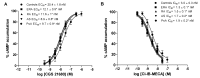
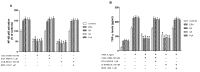
Rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) are chronic inflammatory rheumatic diseases that affect joints, causing debilitating pain and disability. Adenosine receptors (ARs) play a key role in the mechanism of inflammation, and the activation of A2A and A₃AR subtypes is often associated with a reduction of the inflammatory status. The aim of this study was to investigate the involvement of ARs in patients suffering from early-RA (ERA), RA, AS and PsA. Messenger RNA (mRNA) analysis and saturation binding experiments indicated an upregulation of A2A and A₃ARs in lymphocytes obtained from patients when compared with healthy subjects. A2A and A₃AR agonists inhibited nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) activation and reduced inflammatory cytokines release, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6. Moreover, A2A and A₃AR activation mediated a reduction of metalloproteinases (MMP)-1 and MMP-3. The effect of the agonists was abrogated by selective antagonists demonstrating the direct involvement of these receptor subtypes. Taken together, these data confirmed the involvement of ARs in chronic autoimmune rheumatic diseases highlighting the possibility to exploit A2A and A₃ARs as therapeutic targets, with the aim to limit the inflammatory responses usually associated with RA, AS and PsA.
Keywords: adenosine receptors; ankylosing spondylitis; inflammation; psoriatic arthritis; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release.Arthritis Res Ther. 2011;13(6):R197. doi: 10.1186/ar3527. Epub 2011 Dec 6. Arthritis Res Ther. 2011. PMID: 22146575 Free PMC article.
-
Normalization of A2A and A3 adenosine receptor up-regulation in rheumatoid arthritis patients by treatment with anti-tumor necrosis factor alpha but not methotrexate.Arthritis Rheum. 2009 Oct;60(10):2880-91. doi: 10.1002/art.24794. Arthritis Rheum. 2009. PMID: 19790066
-
Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated.Eur J Immunol. 2013 Aug;43(8):2206-16. doi: 10.1002/eji.201343314. Epub 2013 Jun 3. Eur J Immunol. 2013. PMID: 23661562
-
Perspective and Potential of A2A and A3 Adenosine Receptors as Therapeutic Targets for the Treatment of Rheumatoid Arthritis.Curr Pharm Des. 2019;25(26):2859-2874. doi: 10.2174/1381612825666190710111658. Curr Pharm Des. 2019. PMID: 31291875 Review.
-
A2A Adenosine Receptor Agonists and their Potential Therapeutic Applications. An Update.Curr Med Chem. 2018;25(30):3597-3612. doi: 10.2174/0929867325666180313110254. Curr Med Chem. 2018. PMID: 29532748 Review.
Cited by
-
Piperazine- and Piperidine-Containing Thiazolo[5,4-d]pyrimidine Derivatives as New Potent and Selective Adenosine A2A Receptor Inverse Agonists.Pharmaceuticals (Basel). 2020 Jul 24;13(8):161. doi: 10.3390/ph13080161. Pharmaceuticals (Basel). 2020. PMID: 32722122 Free PMC article.
-
Amino-3,5-Dicyanopyridines Targeting the Adenosine Receptors Ranging from Pan Ligands to Combined A1/A2B Partial Agonists.Pharmaceuticals (Basel). 2019 Oct 22;12(4):159. doi: 10.3390/ph12040159. Pharmaceuticals (Basel). 2019. PMID: 31652622 Free PMC article.
-
Ectonucleotidases in Acute and Chronic Inflammation.Front Pharmacol. 2021 Feb 3;11:619458. doi: 10.3389/fphar.2020.619458. eCollection 2020. Front Pharmacol. 2021. PMID: 33613285 Free PMC article. Review.
-
Adenosine A3 agonists reverse neuropathic pain via T cell-mediated production of IL-10.J Clin Invest. 2021 Apr 1;131(7):e139299. doi: 10.1172/JCI139299. J Clin Invest. 2021. PMID: 33621215 Free PMC article.
-
Electroacupuncture inhibits visceral pain via adenosine receptors in mice with inflammatory bowel disease.Purinergic Signal. 2019 Jun;15(2):193-204. doi: 10.1007/s11302-019-09655-4. Epub 2019 Jun 11. Purinergic Signal. 2019. PMID: 31187350 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

