Nerve Growth Factor Signaling from Membrane Microdomains to the Nucleus: Differential Regulation by Caveolins
- PMID: 28338624
- PMCID: PMC5412279
- DOI: 10.3390/ijms18040693
Nerve Growth Factor Signaling from Membrane Microdomains to the Nucleus: Differential Regulation by Caveolins
Abstract
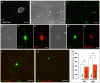
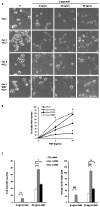
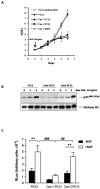
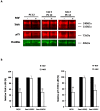
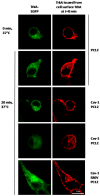
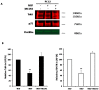
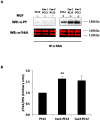
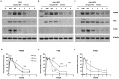
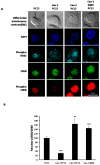
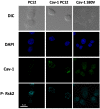
Membrane microdomains or "lipid rafts" have emerged as essential functional modules of the cell, critical for the regulation of growth factor receptor-mediated responses. Herein we describe the dichotomy between caveolin-1 and caveolin-2, structural and regulatory components of microdomains, in modulating proliferation and differentiation. Caveolin-2 potentiates while caveolin-1 inhibits nerve growth factor (NGF) signaling and subsequent cell differentiation. Caveolin-2 does not appear to impair NGF receptor trafficking but elicits prolonged and stronger activation of MAPK (mitogen-activated protein kinase), Rsk2 (ribosomal protein S6 kinase 2), and CREB (cAMP response element binding protein). In contrast, caveolin-1 does not alter initiation of the NGF signaling pathway activation; rather, it acts, at least in part, by sequestering the cognate receptors, TrkA and p75NTR, at the plasma membrane, together with the phosphorylated form of the downstream effector Rsk2, which ultimately prevents CREB phosphorylation. The non-phosphorylatable caveolin-1 serine 80 mutant (S80V), no longer inhibits TrkA trafficking or subsequent CREB phosphorylation. MC192, a monoclonal antibody towards p75NTR that does not block NGF binding, prevents exit of both NGF receptors (TrkA and p75NTR) from lipid rafts. The results presented herein underline the role of caveolin and receptor signaling complex interplay in the context of neuronal development and tumorigenesis.
Keywords: CREB; NGF; PC12; Trk; caveolin; dorsal root ganglion neurons; growth factor signaling; lipid rafts; membrane microdomains; p75NTR; trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein.Mol Cell Biol. 2007 Aug;27(16):5686-98. doi: 10.1128/MCB.01109-06. Epub 2007 Jun 4. Mol Cell Biol. 2007. PMID: 17548467 Free PMC article.
-
Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways.J Biol Chem. 1999 Jan 1;274(1):257-63. doi: 10.1074/jbc.274.1.257. J Biol Chem. 1999. PMID: 9867838
-
Mutual effects of caveolin and nerve growth factor signaling in pig oligodendrocytes.J Neurosci Res. 2010 Feb 15;88(3):572-88. doi: 10.1002/jnr.22235. J Neurosci Res. 2010. PMID: 19795378
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
Nerve growth factor: structure and function.Cell Mol Life Sci. 2001 May;58(5-6):748-59. doi: 10.1007/pl00000898. Cell Mol Life Sci. 2001. PMID: 11437236 Free PMC article. Review.
Cited by
-
Are There Lipid Membrane-Domain Subtypes in Neurons with Different Roles in Calcium Signaling?Molecules. 2023 Dec 2;28(23):7909. doi: 10.3390/molecules28237909. Molecules. 2023. PMID: 38067638 Free PMC article. Review.
-
Expression of BDNF, TrkB, VEGF and CD105 is associated with pelvic lymph node metastasis and prognosis in IB2-stage squamous cell carcinoma.Exp Ther Med. 2019 Dec;18(6):4221-4230. doi: 10.3892/etm.2019.8100. Epub 2019 Oct 14. Exp Ther Med. 2019. PMID: 31777532 Free PMC article.
-
Genome-Wide DNA Methylation Analysis in Male Methamphetamine Users With Different Addiction Qualities.Front Psychiatry. 2020 Oct 23;11:588229. doi: 10.3389/fpsyt.2020.588229. eCollection 2020. Front Psychiatry. 2020. PMID: 33192735 Free PMC article.
-
Understanding the interplay of membrane trafficking, cell surface mechanics, and stem cell differentiation.Semin Cell Dev Biol. 2023 Jan 15;133:123-134. doi: 10.1016/j.semcdb.2022.05.010. Epub 2022 May 28. Semin Cell Dev Biol. 2023. PMID: 35641408 Free PMC article. Review.
-
Proteomics as a Complementary Technique to Characterize Bladder Cancer.Cancers (Basel). 2021 Nov 4;13(21):5537. doi: 10.3390/cancers13215537. Cancers (Basel). 2021. PMID: 34771699 Free PMC article. Review.
References
-
- Tang Z., Scherer P.E., Okamoto T., Song K., Chu C., Kohtz D.S., Nishimoto I., Lodish H.F., Lisanti M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996;271:2255–2261. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

