(R)-(-)-Aloesaponol III 8-Methyl Ether from Eremurus persicus: A Novel Compound against Leishmaniosis
- PMID: 28338625
- PMCID: PMC6154379
- DOI: 10.3390/molecules22040519
(R)-(-)-Aloesaponol III 8-Methyl Ether from Eremurus persicus: A Novel Compound against Leishmaniosis
Abstract
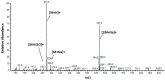
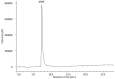
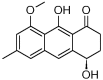
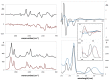
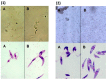
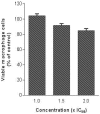
Leishmaniosis is a neglected tropical disease which affects several millions of people worldwide. The current drug therapies are expensive and often lack efficacy, mainly due to the development of parasite resistance. Hence, there is an urgent need for new drugs effective against Leishmania infections. As a part of our ongoing study on the phytochemical characterization and biological investigation of plants used in the traditional medicine of western and central Asia, in the present study, we focused on Eremurus persicus root extract in order to evaluate its potential in the treatment of leishmaniosis. As a result of our study, aloesaponol III 8-methyl ether (ASME) was isolated for the first time from Eremurus persicus root extract, its chemical structure elucidated by means of IR and NMR experiments and the (R) configuration assigned by optical activity measurements: chiroptical aspects were investigated with vibrational circular dichroism (VCD) and electronic circular dichroism (ECD) spectroscopies and DFT (density functional theory) quantum mechanical calculations. Concerning biological investigations, our results clearly proved that (R)-ASME inhibits Leishmania infantum promastigotes viability (IC50 73 µg/mL), inducing morphological alterations and mitochondrial potential deregulation. Moreover, it is not toxic on macrophages at the concentration tested, thus representing a promising molecule against Leishmania infections.
Keywords: (R)-aloesaponol III-8 methyl ether; Eremurus persicus; drug identification; leishmaniosis; plant extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alviano D.S., Barreto A.L.S., de Almeida Dias F., de Almeida Rodrigues I., dos Santos Rosa M.D.S., Alviano C.S., de Araújo Soares R.M. Conventional therapy and promising plant-derived compounds against trypanosomatid parasites. Front. Microbiol. 2012;3:283. doi: 10.3389/fmicb.2012.00283. - DOI - PMC - PubMed
-
- Gaggeri R., Rossi D., Christodoulou M.S., Passarella D., Leoni F., Azzolina O., Collina S. Chiral Flavanones from Amygdalus lycioides Spach: Structural Elucidation and Identification of TNF alpha Inhibitors by Bioactivity-guided Fractionation. Molecules. 2012;17:1665–1674. doi: 10.3390/molecules17021665. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

