Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells
- PMID: 28338627
- PMCID: PMC6153754
- DOI: 10.3390/molecules22040520
Morphology Analysis and Optimization: Crucial Factor Determining the Performance of Perovskite Solar Cells
Abstract
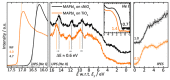
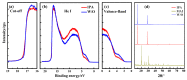
This review presents an overall discussion on the morphology analysis and optimization for perovskite (PVSK) solar cells. Surface morphology and energy alignment have been proven to play a dominant role in determining the device performance. The effect of the key parameters such as solution condition and preparation atmosphere on the crystallization of PVSK, the characterization of surface morphology and interface distribution in the perovskite layer is discussed in detail. Furthermore, the analysis of interface energy level alignment by using X-ray photoelectron spectroscopy and ultraviolet photoelectron spectroscopy is presented to reveals the correlation between morphology and charge generation and collection within the perovskite layer, and its influence on the device performance. The techniques including architecture modification, solvent annealing, etc. were reviewed as an efficient approach to improve the morphology of PVSK. It is expected that further progress will be achieved with more efforts devoted to the insight of the mechanism of surface engineering in the field of PVSK solar cells.
Keywords: CH3NH3PbI3; energy alignment; interface; morphology; perovskite; photovoltaic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

