Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay
- PMID: 28338634
- PMCID: PMC5419789
- DOI: 10.3390/s17040676
Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay
Abstract
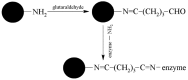
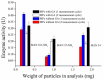
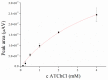
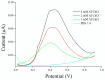
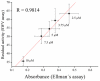
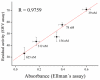
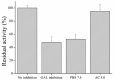
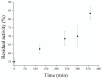
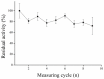
Magnetic particles (MPs) have been widely used in biological applications in recent years as a carrier for various molecules. Their big advantage is in repeated use of immobilized molecules including enzymes. Acetylcholinesterase (AChE) is an enzyme playing crucial role in neurotransmission and the enzyme is targeted by various molecules like Alzheimer's drugs, pesticides and warfare agents. In this work, an electrochemical biosensor having AChE immobilized onto MPs and stabilized through glutaraldehyde (GA) molecule was proposed for assay of the neurotoxic compounds. The prepared nanoparticles were modified by pure AChE and they were used for the measurement anti-Alzheimer's drug galantamine and carbamate pesticide carbofuran with limit of detection 1.5 µM and 20 nM, respectively. All measurements were carried out using screen-printed sensor with carbon working, silver reference, and carbon auxiliary electrode. Standard Ellman's assay was used for validation measurement of both inhibitors. Part of this work was the elimination of reversible inhibitors represented by galantamine from the active site of AChE. For this purpose, we used a lower pH to get the original activity of AChE after inhibition by galantamine. We also observed decarbamylation of the AChE-carbofuran adduct. Influence of organic solvents to AChE as well as repeatability of measurement with MPs with AChE was also established.
Keywords: acetylcholinesterase; carbofuran; electrochemistry; galantamine; magnetic particles; nanomaterial; nanoparticles; screen-printed sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pohanka M. Biosensors containing acetylcholinesterase and butyrylcholinesterase as recognition tools for detection of various compounds. Chem. Pap. 2015;69:4–16. doi: 10.2478/s11696-014-0542-x. - DOI
-
- Kostelnik A., Cegan A., Pohanka M. Electrochemical determination of activity of acetylcholinesterase immobilized on magnetic particles. Int. J. Electrochem. Sci. 2016;11:4840–4849. doi: 10.20964/2016.06.39. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

