Evolution of caspase-mediated cell death and differentiation: twins separated at birth
- PMID: 28338655
- PMCID: PMC5520454
- DOI: 10.1038/cdd.2017.37
Evolution of caspase-mediated cell death and differentiation: twins separated at birth
Abstract
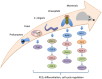
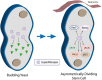
The phenotypic and biochemical similarities between caspase-mediated apoptosis and cellular differentiation are striking. They include such diverse phenomenon as mitochondrial membrane perturbations, cytoskeletal rearrangements and DNA fragmentation. The parallels between the two disparate processes suggest some common ancestry and highlight the paradoxical nature of the death-centric view of caspases. That is, what is the driving selective pressure that sustains death-inducing proteins throughout eukaryotic evolution? Plausibly, caspase function may be rooted in a primordial non-death function, such as cell differentiation, and was co-opted for its role in programmed cell death. This review will delve into the links between caspase-mediated apoptosis and cell differentiation and examine the distinguishing features of these events. More critically, we chronicle the evolutionary origins of caspases and propose that caspases may have held an ancient role in mediating the fidelity of cell division/differentiation through its effects on proteostasis and protein quality control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kumar S. Caspase function in programmed cell death. Cell Death Diff 2007; 14: 32–43. - PubMed
-
- Fernando P, Megeney LA. Is caspase-dependent apoptosis only cell differentiation taken to the extreme? FASEB J 2007; 21: 8–17. - PubMed
-
- Dick SA, Megeney LA. Cell death proteins: an evolutionary role in cellular adaptation before the advent of apoptosis. Bioessays 2013; 35: 974–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

