A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization
- PMID: 28338689
- PMCID: PMC5576532
- DOI: 10.1038/nchem.2664
A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization
Abstract
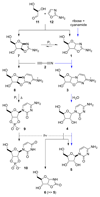
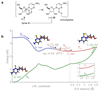
Previous research has identified ribose aminooxazoline as a potential intermediate in the prebiotic synthesis of the pyrimidine nucleotides with remarkable properties. It crystallizes spontaneously from reaction mixtures, with an enhanced enantiomeric excess if initially enantioenriched, which suggests that reservoirs of this compound might have accumulated on the early Earth in an optically pure form. Ribose aminooxazoline can be converted efficiently into α-ribocytidine by way of 2,2'-anhydroribocytidine, although anomerization to β-ribocytidine by ultraviolet irradiation is extremely inefficient. Our previous work demonstrated the synthesis of pyrimidine β-ribonucleotides, but at the cost of ignoring ribose aminooxazoline, using arabinose aminooxazoline instead. Here we describe a long-sought route through ribose aminooxazoline to the pyrimidine β-ribonucleosides and their phosphate derivatives that involves an extraordinarily efficient photoanomerization of α-2-thioribocytidine. In addition to the canonical nucleosides, our synthesis accesses β-2-thioribouridine, a modified nucleoside found in transfer RNA that enables both faster and more-accurate nucleic acid template-copying chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions.Nature. 2009 May 14;459(7244):239-42. doi: 10.1038/nature08013. Nature. 2009. PMID: 19444213
-
2'-C-branched ribonucleosides. 2. Synthesis of 2'-C-beta-trifluoromethyl pyrimidine ribonucleosides.Org Lett. 2001 Apr 5;3(7):1025-8. doi: 10.1021/ol0155687. Org Lett. 2001. PMID: 11277786
-
On the prebiotic synthesis of ribonucleotides: photoanomerisation of cytosine nucleosides and nucleotides revisited.Chembiochem. 2007 Jul 9;8(10):1170-9. doi: 10.1002/cbic.200700098. Chembiochem. 2007. PMID: 17549787
-
Practical synthesis of 4'-thioribonucleosides starting from D-ribose.Curr Protoc Nucleic Acid Chem. 2014 Dec 12;59:14.12.1-19. doi: 10.1002/0471142700.nc1412s59. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25501591 Review.
-
Ribonucleotides.Cold Spring Harb Perspect Biol. 2010 Apr;2(4):a005439. doi: 10.1101/cshperspect.a005439. Epub 2010 Mar 10. Cold Spring Harb Perspect Biol. 2010. PMID: 20452951 Free PMC article. Review.
Cited by
-
Stem Life: A Framework for Understanding the Prebiotic-Biotic Transition.J Mol Evol. 2024 Oct;92(5):539-549. doi: 10.1007/s00239-024-10201-z. Epub 2024 Sep 8. J Mol Evol. 2024. PMID: 39244680 Free PMC article.
-
Prebiotic synthesis of dihydrouridine by photoreduction of uridine in formamide.Chem Commun (Camb). 2024 Jul 4;60(55):7081-7084. doi: 10.1039/d4cc01823k. Chem Commun (Camb). 2024. PMID: 38896044 Free PMC article.
-
Enhanced Nonenzymatic RNA Copying with 2-Aminoimidazole Activated Nucleotides.J Am Chem Soc. 2017 Feb 8;139(5):1810-1813. doi: 10.1021/jacs.6b13148. Epub 2017 Jan 24. J Am Chem Soc. 2017. PMID: 28117989 Free PMC article.
-
Illuminating Life's Origins: UV Photochemistry in Abiotic Synthesis of Biomolecules.J Am Chem Soc. 2021 May 19;143(19):7219-7236. doi: 10.1021/jacs.1c01839. Epub 2021 Apr 21. J Am Chem Soc. 2021. PMID: 33880920 Free PMC article. Review.
-
Shielding from UV Photodamage: Implications for Surficial Origins of Life Chemistry on the Early Earth.ACS Earth Space Chem. 2021 Feb 18;5(2):239-246. doi: 10.1021/acsearthspacechem.0c00270. Epub 2021 Jan 29. ACS Earth Space Chem. 2021. PMID: 36317066 Free PMC article.
References
-
- Sanchez RA, Orgel LE. Studies in prebiotic synthesis. Synthesis and photoanomerization of pyrimidine nucleosides. J Mol Biol. 1970;47:531–543. - PubMed
-
- Powner MW, et al. On the prebiotic synthesis of ribonucleotides: photoanomerisation of cytosine nucleosides and nucleotides revisited. ChemBioChem. 2007;8:1170–1179. - PubMed
-
- Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. - PubMed
-
- Anastasi C, Crowe MA, Powner MW, Sutherland JD. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew Chem Int Ed. 2006;45:6176–6179. - PubMed
-
- Powner MW, Sutherland JD. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew Chem Int Ed. 2010;49:4641–4643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/318488390
- PubChem-Substance/318488401
- PubChem-Substance/318488407
- PubChem-Substance/318488408
- PubChem-Substance/318488409
- PubChem-Substance/318488410
- PubChem-Substance/318488411
- PubChem-Substance/318488412
- PubChem-Substance/318488413
- PubChem-Substance/318488391
- PubChem-Substance/318488392
- PubChem-Substance/318488393
- PubChem-Substance/318488394
- PubChem-Substance/318488395
- PubChem-Substance/318488396
- PubChem-Substance/318488397
- PubChem-Substance/318488398
- PubChem-Substance/318488399
- PubChem-Substance/318488400
- PubChem-Substance/318488402
- PubChem-Substance/318488403
- PubChem-Substance/318488404
- PubChem-Substance/318488405
- PubChem-Substance/318488406
LinkOut - more resources
Full Text Sources
Other Literature Sources

