RIN4 recruits the exocyst subunit EXO70B1 to the plasma membrane
- PMID: 28338727
- PMCID: PMC5853926
- DOI: 10.1093/jxb/erx007
RIN4 recruits the exocyst subunit EXO70B1 to the plasma membrane
Abstract
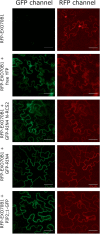
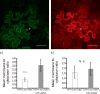
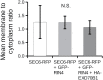
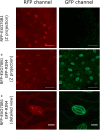
The exocyst is a conserved vesicle-tethering complex with principal roles in cell polarity and morphogenesis. Several studies point to its involvement in polarized secretion during microbial pathogen defense. In this context, we have found an interaction between the Arabidopsis EXO70B1 exocyst subunit, a protein which was previously associated with both the defense response and autophagy, and RPM1 INTERACTING PROTEIN 4 (RIN4), the best studied member of the NOI protein family and a known regulator of plant defense pathways. Interestingly, fragments of RIN4 mimicking the cleavage caused by the Pseudomonas syringae effector protease, AvrRpt2, fail to interact strongly with EXO70B1. We observed that transiently expressed RIN4, but not the plasma membrane (PM) protein aquaporin PIP2, recruits EXO70B1 to the PM. Unlike EXO70B1, RIN4 does not recruit the core exocyst subunit SEC6 to the PM under these conditions. Furthermore, the AvrRpt2 effector protease delivered by P. syringae is able to release both RIN4 and EXO70B1 to the cytoplasm. We present a model for how RIN4 might regulate the localization and putative function of EXO70B1 and speculate on the role the AvrRpt2 protease might have in the regulation of this defense response.
Keywords: Autophagy; EXO70B1; EXO70B2; RIN4; exocyst; plant immunity; secretion.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures





Similar articles
-
The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation.Proc Natl Acad Sci U S A. 2005 May 3;102(18):6496-501. doi: 10.1073/pnas.0500792102. Epub 2005 Apr 21. Proc Natl Acad Sci U S A. 2005. PMID: 15845764 Free PMC article.
-
Membrane release and destabilization of Arabidopsis RIN4 following cleavage by Pseudomonas syringae AvrRpt2.Mol Plant Microbe Interact. 2005 Dec;18(12):1258-68. doi: 10.1094/MPMI-18-1258. Mol Plant Microbe Interact. 2005. PMID: 16478045
-
Separable fragments and membrane tethering of Arabidopsis RIN4 regulate its suppression of PAMP-triggered immunity.Plant Cell. 2011 Oct;23(10):3798-811. doi: 10.1105/tpc.111.088708. Epub 2011 Oct 7. Plant Cell. 2011. PMID: 21984695 Free PMC article.
-
Functions of RPM1-interacting protein 4 in plant immunity.Planta. 2021 Jan 3;253(1):11. doi: 10.1007/s00425-020-03527-7. Planta. 2021. PMID: 33389186 Review.
-
Regulated Disorder: Posttranslational Modifications Control the RIN4 Plant Immune Signaling Hub.Mol Plant Microbe Interact. 2019 Jan;32(1):56-64. doi: 10.1094/MPMI-07-18-0212-FI. Epub 2018 Nov 12. Mol Plant Microbe Interact. 2019. PMID: 30418084 Free PMC article. Review.
Cited by
-
OsExo70B1 Positively Regulates Disease Resistance to Magnaporthe oryzae in Rice.Int J Mol Sci. 2020 Sep 25;21(19):7049. doi: 10.3390/ijms21197049. Int J Mol Sci. 2020. PMID: 32992695 Free PMC article.
-
Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth.Plants (Basel). 2020 Aug 26;9(9):1098. doi: 10.3390/plants9091098. Plants (Basel). 2020. PMID: 32859043 Free PMC article. Review.
-
The Upregulated Expression of the Citrus RIN4 Gene in HLB Diseased Citrus Aids Candidatus Liberibacter Asiaticus Infection.Int J Mol Sci. 2022 Jun 23;23(13):6971. doi: 10.3390/ijms23136971. Int J Mol Sci. 2022. PMID: 35805971 Free PMC article.
-
Exchanging missives and missiles: the roles of extracellular vesicles in plant-pathogen interactions.J Exp Bot. 2017 Nov 28;68(20):5411-5414. doi: 10.1093/jxb/erx369. J Exp Bot. 2017. PMID: 29190393 Free PMC article.
-
A conserved glutamate residue in RPM1-INTERACTING PROTEIN4 is ADP-ribosylated by the Pseudomonas effector AvrRpm2 to activate RPM1-mediated plant resistance.Plant Cell. 2022 Nov 29;34(12):4950-4972. doi: 10.1093/plcell/koac286. Plant Cell. 2022. PMID: 36130293 Free PMC article.
References
-
- An Q, Hückelhoven R, Kogel KH, van Bel AJ. 2006. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cellular Microbiology 8, 1009–1019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

