Recombination-Mediated Host Adaptation by Avian Staphylococcus aureus
- PMID: 28338786
- PMCID: PMC5469444
- DOI: 10.1093/gbe/evx037
Recombination-Mediated Host Adaptation by Avian Staphylococcus aureus
Abstract
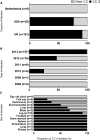
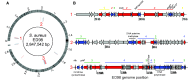
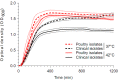
Staphylococcus aureus are globally disseminated among farmed chickens causing skeletal muscle infections, dermatitis, and septicaemia. The emergence of poultry-associated lineages has involved zoonotic transmission from humans to chickens but questions remain about the specific adaptations that promote proliferation of chicken pathogens. We characterized genetic variation in a population of genome-sequenced S. aureus isolates of poultry and human origin. Genealogical analysis identified a dominant poultry-associated sequence cluster within the CC5 clonal complex. Poultry and human CC5 isolates were significantly distinct from each other and more recombination events were detected in the poultry isolates. We identified 44 recombination events in 33 genes along the branch extending to the poultry-specific CC5 cluster, and 47 genes were found more often in CC5 poultry isolates compared with those from humans. Many of these gene sequences were common in chicken isolates from other clonal complexes suggesting horizontal gene transfer among poultry associated lineages. Consistent with functional predictions for putative poultry-associated genes, poultry isolates showed enhanced growth at 42 °C and greater erythrocyte lysis on chicken blood agar in comparison with human isolates. By combining phenotype information with evolutionary analyses of staphylococcal genomes, we provide evidence of adaptation, following a human-to-poultry host transition. This has important implications for the emergence and dissemination of new pathogenic clones associated with modern agriculture.
Keywords: Staphylococcus; evolution; genomics; poultry infection; recombination.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures


References
-
- Abdalrahman LS, Stanley A, Wells H, Fakhr MK. 2015. Isolation, virulence, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains from Oklahoma retail poultry meats. Int J Environ Res Public Health 12:6148–6161. - PMC - PubMed
-
- Anukool U, et al. 2011. Meticillin-resistant Staphylococcus aureus in pigs from Thailand. Int J Antimicrob Agents 38:86–87. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/L015080/1/MRC_/Medical Research Council/United Kingdom
- BB/I02464X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0801929/MRC_/Medical Research Council/United Kingdom
- BB/I013873/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K00638X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

