Conserved Patterns of Sex Chromosome Dosage Compensation in the Lepidoptera (WZ/ZZ): Insights from a Moth Neo-Z Chromosome
- PMID: 28338816
- PMCID: PMC5381563
- DOI: 10.1093/gbe/evx039
Conserved Patterns of Sex Chromosome Dosage Compensation in the Lepidoptera (WZ/ZZ): Insights from a Moth Neo-Z Chromosome
Abstract
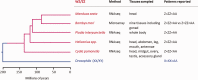
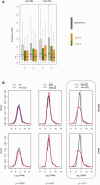
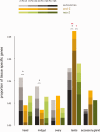
Where previously described, patterns of sex chromosome dosage compensation in the Lepidoptera (moths and butterflies) have several unusual characteristics. Other female-heterogametic (ZW/ZZ) species exhibit female Z-linked expression that is reduced compared with autosomal expression and male Z expression. In the Lepidoptera, however, Z expression typically appears balanced between sexes but overall reduced relative to autosomal expression, that is Z ≈ ZZ < AA. This pattern is not easily reconciled with theoretical expectations for the evolution of sex chromosome dosage compensation. Moreover, conflicting results linger due to discrepancies in data analyses and tissues sampled among lepidopterans. To address these issues, we performed RNA-seq to analyze sex chromosome dosage compensation in the codling moth, Cydia pomonella, which is a species from the earliest diverging lepidopteran lineage yet examined for dosage compensation and has a neo-Z chromosome resulting from an ancient Z:autosome fusion. While supported by intraspecific analyses, the Z ≈ ZZ < AA pattern was further evidenced by comparative study using autosomal orthologs of C. pomonella neo-Z genes in outgroup species. In contrast, dosage compensation appears to be absent in reproductive tissues. We thus argue that inclusion of reproductive tissues may explain the incongruence from a prior study on another moth species and that patterns of dosage compensation are likely conserved in the Lepidoptera. Notably, this pattern appears convergent with patterns in eutherian mammals (X ≈ XX < AA). Overall, our results contribute to the notion that the Lepidoptera present challenges both to classical theories regarding the evolution of sex chromosome dosage compensation and the emerging view of the association of dosage compensation with sexual heterogamety.
Keywords: Cydia pomonella; Lepidoptera; neo-Z chromosome; sex chromosome dosage compensation; sex-biased gene expression.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

