Quality of life with cediranib in relapsed ovarian cancer: The ICON6 phase 3 randomized clinical trial
- PMID: 28339098
- PMCID: PMC5516140
- DOI: 10.1002/cncr.30657
Quality of life with cediranib in relapsed ovarian cancer: The ICON6 phase 3 randomized clinical trial
Abstract
Background: The ICON6 trial showed that cediranib, an oral inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, improved clinical outcomes for patients with platinum-sensitive relapsed ovarian cancer when it was used with chemotherapy and was continued as maintenance therapy. This study describes health-related quality of life (QOL) during the first year of treatment.
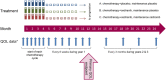
Methods: Four hundred fifty-six women were randomly allocated to receive standard chemotherapy only, chemotherapy with concurrent cediranib, or chemotherapy with cediranib administered concurrently and continued as maintenance. Patients completed QOL questionnaires until disease progression every 3 weeks during chemotherapy and then every 6 weeks to 1 year. Patients alive with disease progression completed a QOL form 1 year after randomization. The primary QOL endpoint was the global score from the Quality of Life Questionnaire Core 30 (of the European Organization for Research and Treatment of Cancer) at 1 year, with the standard chemotherapy group compared with the concurrent-maintenance cediranib group.
Results: The rate of questionnaire compliance was 90% at the baseline and 76% at 1 year and was similar across the 3 groups. The mean global QOL score at 1 year was 62.6 points for the standard chemotherapy group and 68.7 points for the concurrent-maintenance group (+4.5; 95% confidence interval, -2.0 to 11.0; P = .18). Sensitivity analyses suggested that this finding was robust to the effect of missing data, and the improvement became statistically significant after adjustments for self-reported diarrhea.
Conclusions: The 6th study by the International Collaboration in Ovarian Neoplasm (ICON6) showed a significant improvement in progression-free survival with cediranib as concurrent and maintenance therapy. No QOL detriment with cediranib was found 1 year after treatment was commenced. The maintenance of QOL along with prolonged cancer control suggests that cediranib has a valuable role in the treatment of relapsed ovarian cancer. Cancer 2017;123:2752-61. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Keywords: chemotherapy; health-related quality of life; ovarian cancer.
© 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures


References
-
- Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient‐reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249‐4255. - PubMed
-
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925‐2934. - PubMed
-
- Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor–induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841‐844. - PubMed
-
- Eskander RN, Tewari KS. Incorporation of anti‐angiogenesis therapy in the management of advanced ovarian carcinoma—mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol. 2014;132:496‐505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

