Morphological and morphometric specializations of the lung of the Andean goose, Chloephaga melanoptera: A lifelong high-altitude resident
- PMID: 28339478
- PMCID: PMC5365123
- DOI: 10.1371/journal.pone.0174395
Morphological and morphometric specializations of the lung of the Andean goose, Chloephaga melanoptera: A lifelong high-altitude resident
Abstract
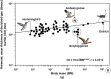
High altitude flight in rarefied, extremely cold and hypoxic air is a very challenging activity. Only a few species of birds can achieve it. Hitherto, the structure of the lungs of such birds has not been studied. This is because of the rarity of such species and the challenges of preparing well-fixed lung tissue. Here, it was posited that in addition to the now proven physiological adaptations, high altitude flying birds will also have acquired pulmonary structural adaptations that enable them to obtain the large amounts of oxygen (O2) needed for flight at high elevation, an environment where O2 levels are very low. The Andean goose (Chloephaga melanoptera) normally resides at altitudes above 3000 meters and flies to elevations as high as 6000 meters where O2 becomes limiting. In this study, its lung was morphologically- and morphometrically investigated. It was found that structurally the lungs are exceptionally specialized for gas exchange. Atypically, the infundibulae are well-vascularized. The mass-specific volume of the lung (42.8 cm3.kg-1), the mass-specific respiratory surface area of the blood-gas (tissue) barrier (96.5 cm2.g-1) and the mass-specific volume of the pulmonary capillary blood (7.44 cm3.kg-1) were some of the highest values so far reported in birds. The pulmonary structural specializations have generated a mass-specific total (overall) pulmonary morphometric diffusing capacity of the lung for oxygen (DLo2) of 0.119 mlO2.sec-1.mbar-1.kg-1, a value that is among some of the highest ones in birds that have been studied. The adaptations of the lung of the Andean goose possibly produce the high O2 conductance needed to live and fly at high altitude.
Conflict of interest statement
Figures
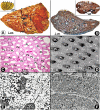
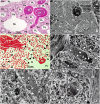
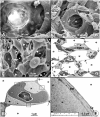


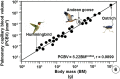


References
-
- Gehr P, Mwangi DK, Ammann A, Maloiy GMO, Taylor CR, Weibel ER. Design of the mammalian respiratory system: V. Scaling morphometric diffusing capacity to body mass: wild and domestic animals. Respir Physiol. 1981; 44:61–86. - PubMed
-
- White CR, Kearney MR. Metabolic scaling in animals: methods, empirical results and theoretical explanations. Comp Physiol. 2014; 4:231–256. - PubMed
-
- Black CP, Tenney SM. Oxygen transport during progressive hypoxia in high altitude and sea level water-fowl. Respir Physiol. 1980; 39:217–239. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

