Analysis of professional competencies for the clinical research data management profession: implications for training and professional certification
- PMID: 28339721
- PMCID: PMC6080682
- DOI: 10.1093/jamia/ocw179
Analysis of professional competencies for the clinical research data management profession: implications for training and professional certification
Abstract
Objective: To assess and refine competencies for the clinical research data management profession.
Materials and methods: Based on prior work developing and maintaining a practice standard and professional certification exam, a survey was administered to a captive group of clinical research data managers to assess professional competencies, types of data managed, types of studies supported, and necessary foundational knowledge.
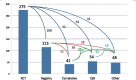
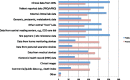
Results: Respondents confirmed a set of 91 professional competencies. As expected, differences were seen in job tasks between early- to mid-career and mid- to late-career practitioners. Respondents indicated growing variability in types of studies for which they managed data and types of data managed.
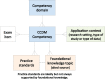
Discussion: Respondents adapted favorably to the separate articulation of professional competencies vs foundational knowledge. The increases in the types of data managed and variety of research settings in which data are managed indicate a need for formal education in principles and methods that can be applied to different research contexts (ie, formal degree programs supporting the profession), and stronger links with the informatics scientific discipline, clinical research informatics in particular.
Conclusion: The results document the scope of the profession and will serve as a foundation for the next revision of the Certified Clinical Data Manager TM exam. A clear articulation of professional competencies and necessary foundational knowledge could inform the content of graduate degree programs or tracks in areas such as clinical research informatics that will develop the current and future clinical research data management workforce.
Keywords: clinical data management; clinical research informatics; professional competencies.
© The Author 2017. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures
Similar articles
-
Analysis of Professional Competencies for the Clinical Research Data Management Profession.Stud Health Technol Inform. 2020 Jun 16;270:1199-1200. doi: 10.3233/SHTI200361. Stud Health Technol Inform. 2020. PMID: 32570578
-
Core informatics competencies for clinical and translational scientists: what do our customers and collaborators need to know?J Am Med Inform Assoc. 2016 Jul;23(4):835-9. doi: 10.1093/jamia/ocw047. Epub 2016 Apr 27. J Am Med Inform Assoc. 2016. PMID: 27121608 Free PMC article.
-
Domains, tasks, and knowledge for clinical informatics subspecialty practice: results of a practice analysis.J Am Med Inform Assoc. 2019 Jul 1;26(7):586-593. doi: 10.1093/jamia/ocz051. J Am Med Inform Assoc. 2019. PMID: 31037303 Free PMC article.
-
Updating professional competencies in health informatics: A scoping review and consultation with subject matter experts.Int J Med Inform. 2023 Feb;170:104969. doi: 10.1016/j.ijmedinf.2022.104969. Epub 2022 Dec 20. Int J Med Inform. 2023. PMID: 36572000
-
Global health informatics education.Stud Health Technol Inform. 2000;57:3-14. Stud Health Technol Inform. 2000. PMID: 10947666 Review.
Cited by
-
Comparing Medical Record Abstraction (MRA) error rates in an observational study to pooled rates identified in the data quality literature.BMC Med Res Methodol. 2024 Dec 18;24(1):304. doi: 10.1186/s12874-024-02424-x. BMC Med Res Methodol. 2024. PMID: 39695394 Free PMC article.
-
Informing training needs for the revised certified clinical data manager (CCDMTM) exam: analysis of results from the previous exam.JAMIA Open. 2022 Feb 22;5(1):ooac010. doi: 10.1093/jamiaopen/ooac010. eCollection 2022 Apr. JAMIA Open. 2022. PMID: 35274085 Free PMC article.
-
Understanding enterprise data warehouses to support clinical and translational research: enterprise information technology relationships, data governance, workforce, and cloud computing.J Am Med Inform Assoc. 2022 Mar 15;29(4):671-676. doi: 10.1093/jamia/ocab256. J Am Med Inform Assoc. 2022. PMID: 35289370 Free PMC article.
-
Informatics infrastructure in a rural pediatric clinical trials network: Matching specific clinical research needs with best practices and industry guidelines.Contemp Clin Trials. 2023 Mar;126:107110. doi: 10.1016/j.cct.2023.107110. Epub 2023 Feb 3. Contemp Clin Trials. 2023. PMID: 36738915 Free PMC article.
-
From clinical data management to clinical data science: Time for a new educational model.Clin Transl Sci. 2023 Aug;16(8):1340-1351. doi: 10.1111/cts.13545. Epub 2023 May 24. Clin Transl Sci. 2023. PMID: 37587756 Free PMC article.
References
-
- McBride R, Singer SW, Introduction [to the 1995 Clinical Data Management Special Issue of Controlled Clinical Trials]. Controlled Clinical Trials. 1995;16:1S–3S.
-
- Society for Clinical Data Management . Good Clinical Data Management Practices (GCDMP), October 2013. http://www.scdm.org. Accessed January 20, 2017.
-
- Bloom BS, Krathwohl DR. Taxonomy of educational objectives: the classification of educational goals, by a committee of college and university examiners. Handbook I: Cognitive Domain. New York, NY: Longmans, Green; 1956.
-
- Anderson LW, Krathwohl DR, Airasian PW, Cruikshank KA, Mayer RE, Pintrich PR et al., eds. A Taxonomy for Learning, Teaching, and Assessing: A Revision of Bloom’s Taxonomy of Educational Objectives. Boston, MA: Allyn & Bacon; 2001.
-
- Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;63:9s. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials