The transcription factor Olig2 is important for the biology of diffuse intrinsic pontine gliomas
- PMID: 28339768
- PMCID: PMC5570182
- DOI: 10.1093/neuonc/now299
The transcription factor Olig2 is important for the biology of diffuse intrinsic pontine gliomas
Abstract
Background: Diffuse intrinsic pontine glioma (DIPG) is a high-grade brainstem glioma of children with dismal prognosis. There is no single unifying model about the cell of origin of DIPGs. Proliferating cells in the developing human and mouse pons, the site of DIPGs, express neural stem/progenitor cell (NPC) markers, including Sox2, nestin, vimentin, Olig2, and glial fibrillary acidic protein, in an overlapping and non-overlapping manner, suggesting progenitor cell heterogeneity in the pons. It is thought that during a restricted window of postnatal pons development, a differentiation block caused by genetic/epigenetic changes leads to unrestrained progenitor proliferation and DIPG development. Nearly 80% of DIPGs harbor a mutation in the H3F3A or the related HIST1H3B gene. Supporting the impaired differentiation model, NPCs derived from human induced pluripotent stem cells expressing the H3F3A mutation showed complete differentiation block. However, the mechanisms regulating an altered differentiation program in DIPG are unknown.
Methods: We established syngeneic serum-dependent and independent primary DIPG lines, performed molecular characterization of DIPG lines in vitro and in an orthotopic xenograft model, and used small hairpin RNA to examine Olig2 function in DIPG.
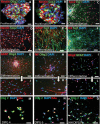
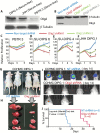
Results: The transcription factor Olig2 is highly expressed in 70%-80% of DIPGs. Here we report that Olig2 expression and DIPG differentiation are mutually exclusive events in vitro, and only DIPG cells that retained Olig2 in vitro formed robust Olig2-positive brainstem glioma with 100% penetrance in a xenograft model.
Conclusion: Our results indicate Olig2 as an onco-requisite factor in DIPG and propose investigation of Olig2 target genes as novel candidates in DIPG therapy.
Keywords: DIPG; Olig2; differentiation.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures




References
-
- Fisher PG, Breiter SN, Carson BS, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89(7):1569–1576. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

