VDAC1 is a molecular target in glioblastoma, with its depletion leading to reprogrammed metabolism and reversed oncogenic properties
- PMID: 28339833
- PMCID: PMC5570220
- DOI: 10.1093/neuonc/now297
VDAC1 is a molecular target in glioblastoma, with its depletion leading to reprogrammed metabolism and reversed oncogenic properties
Erratum in
-
Corrigenda.Neuro Oncol. 2017 Nov 29;19(12):1701. doi: 10.1093/neuonc/nox102. Neuro Oncol. 2017. PMID: 28655211 Free PMC article. No abstract available.
Abstract
Background: Glioblastoma (GBM), an aggressive brain tumor with frequent relapses and a high mortality, still awaits an effective treatment. Like many cancers, GBM cells acquire oncogenic properties, including metabolic reprogramming, vital for growth. As such, tumor metabolism is an emerging avenue for cancer therapy. One relevant target is the voltage-dependent anion channel 1 (VDAC1), a mitochondrial protein controlling cell energy and metabolic homeostasis.
Methods: We used VDAC1-specific short interfering (si)RNA (si-VDAC1) to treat GBM cell lines and subcutaneous or intracranial-orthotopic GBM xenograft mouse models. Tumors were monitored using MRI, immunohistochemistry, immunoblotting, immunofluorescence, quantitative real-time PCR, transcription factor expression, and DNA microarray analyses.
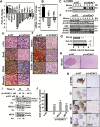
Results: Silencing VDAC1 expression using si-VDAC1 in 9 glioblastoma-related cell lines, including patient-derived cells, led to marked decreases in VDAC1 levels and cell growth. Using si-VDAC1 in subcutaneous or intracranial-orthotopic GBM models inhibited tumor growth and reversed oncogenic properties, such as reprogrammed metabolism, stemness, angiogenesis, epithelial-mesenchymal transition, and invasiveness. In cells in culture, si-VDAC1 inhibits cancer neurosphere formation and, in tumors, targeted cancer stem cells, leading to their differentiation into neuronal-like cells. These VDAC1 depletion-mediated effects involved alterations in transcription factors regulating signaling pathways associated with cancer hallmarks.
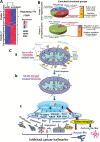
Conclusion: VDAC1 offers a target for GBM treatment, allowing for attacks on the interplay between metabolism and oncogenic signaling networks, leading to tumor cell differentiation into neuron- and astrocyte-like cells. Simultaneously attacking all of these processes, VDAC1 depletion overcame GBM heterogeneity and can replace several anticancer drugs that separately target angiogenesis, proliferation, or metabolism.
Keywords: glioblastoma; metabolism; mitochondria; siRNA; voltage-dependent anion channel.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

