Uterine artery leptin receptors during the ovarian cycle and pregnancy regulate angiogenesis in ovine uterine artery endothelial cells†
- PMID: 28339937
- PMCID: PMC5819836
- DOI: 10.1093/biolre/iox008
Uterine artery leptin receptors during the ovarian cycle and pregnancy regulate angiogenesis in ovine uterine artery endothelial cells†
Erratum in
-
Erratum: Uterine artery leptin receptors during the ovarian cycle and pregnancy regulate angiogenesis in ovine uterine artery endothelial cells.Biol Reprod. 2017 Apr 1;96(4):938. doi: 10.1093/biolre/iox027. Biol Reprod. 2017. PMID: 28525922 Free PMC article. No abstract available.
Abstract
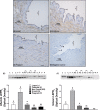
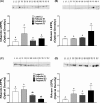
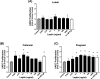
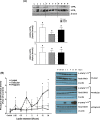
Leptin regulates body weight, reproductive functions, blood pressure, endothelial function, and fetoplacental angiogenesis. Compared to the luteal phase, the follicular phase and pregnancy are physiological states of elevated estrogen, angiogenesis, and uterine blood flow (UBF). Little is known concerning regulation of uterine artery (UA) angiogenesis by leptin and its receptors. We hypothesized that (1) ex vivo expression of leptin receptors (LEPR) in UA endothelium (UAendo) and UA vascular smooth muscle (UAvsm) is elevated in pregnant versus nonpregnant (Luteal and Follicular) sheep; (2) in vitro leptin treatments differentially modulate mitogenesis in uterine artery endothelial cells from pregnant (P-UAECs) more than in nonpregnant (NP-UAECs) ewes; and (3) LEPR are upregulated in P-UAECs versus NP-UAECs in association with leptin activation of phospho-STAT3 signaling. Local UA adaptations were evaluated using a unilateral pregnant sheep model where prebreeding uterine horn isolation (nongravid) restricted gravidity to one horn. Immunolocalization revealed LEPR in UAendo and UAvsm from pregnant and nonpregnant sheep. Contrary to our hypothesis, western analysis revealed that follicular UAendo and UAvsm LEPR were greater than luteal, nongravid, gravid, and control pregnant. Compared to pregnant groups, LEPR were elevated in renal artery endothelium of follicular and luteal sheep. Leptin treatment significantly increased mitogenesis in follicular phase NP-UAECs and P-UAECs, but not luteal phase NP-UAECs. Although UAEC expression of LEPR was similar between groups, leptin treatment only activated phospho-STAT3 in follicular NP-UAECs and P-UAECs. Thus, leptin may play an angiogenic role particularly in preparation for the increased UBF during the periovulatory period and subsequently to meet the demands of the growing fetus.
Keywords: angiogenesis; blood flow; estrogen; leptin; pregnancy; sheep; uterine artery.
© The Authors 2017. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998; 395:763–770. - PubMed
-
- Mark AL, Correia ML, Rahmouni K, Haynes WG. Loss of leptin actions in obesity: two concepts with cardiovascular implications. Clin Exp Hypertens 2004; 26:629–636. - PubMed
-
- Conway GS, Jacobs HS. Leptin: a hormone of reproduction. Hum Reprod 1997; 12:633–635. - PubMed
-
- Winters B, Mo Z, Brooks-Asplund E, Kim S, Shoukas A, Li D, Nyhan D, Berkowitz DE. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol 2000; 89:2382–2390. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

