Evaluating the Patterns of Aging-Related Tau Astrogliopathy Unravels Novel Insights Into Brain Aging and Neurodegenerative Diseases
- PMID: 28340083
- PMCID: PMC6251691
- DOI: 10.1093/jnen/nlx007
Evaluating the Patterns of Aging-Related Tau Astrogliopathy Unravels Novel Insights Into Brain Aging and Neurodegenerative Diseases
Abstract
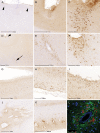
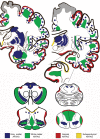
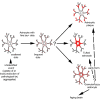
The term "aging-related tau astrogliopathy" (ARTAG) describes pathological accumulation of abnormally phosphorylated tau protein in astrocytes. We evaluated the correlates of ARTAG types (i.e., subpial, subependymal, white and gray matter, and perivascular) in different neuroanatomical regions. Clinical, neuropathological, and genetic (eg, APOE ε4 allele, MAPT H1/H2 haplotype) data from 628 postmortem brains from subjects were investigated; most of the patients had been longitudinally followed at the University of Pennsylvania. We found that (i) the amygdala is a hotspot for all ARTAG types; (ii) age at death, male sex, and presence of primary frontotemporal lobar degeneration (FTLD) tauopathy are significantly associated with ARTAG; (iii) age at death, greater degree of brain atrophy, ventricular enlargement, and Alzheimer disease (AD)-related variables are associated with subpial, white matter, and perivascular ARTAG types; (iv) AD-related variables are associated particularly with lobar white matter ARTAG; and (v) gray matter ARTAG in primary FTLD-tauopathies appears in areas without neuronal tau pathology. We provide a reference map of ARTAG types and propose at least 5 constellations of ARTAG. Furthermore, we propose a conceptual link between primary FTLD-tauopathy and ARTAG-related astrocytic tau pathologies. Our observations serve as a basis for etiological stratification and definition of progression patterns of ARTAG.
Keywords: ARTAG; Aging-related tau astrogliopathy; Alzheimer disease; Chronic traumatic encephalopathy; Dementia; Tau; Tauopathy.
© 2017 American Association of Neuropathologists, Inc. All rights reserved.
Figures




References
-
- Kovacs GG, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 2013;126:365–84 - PubMed
-
- Kovacs GG, Molnar K, Laszlo L, et al. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 2011;122:205–22 - PubMed
-
- Munoz DG, Woulfe J, Kertesz A.. Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol 2007;114:347–57 - PubMed
-
- Ikeda K, Akiyama H, Kondo H, et al. Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol 1995;90:620–5 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

