Activation mechanisms of αVβ3 integrin by binding to fibronectin: A computational study
- PMID: 28340512
- PMCID: PMC5441423
- DOI: 10.1002/pro.3163
Activation mechanisms of αVβ3 integrin by binding to fibronectin: A computational study
Abstract
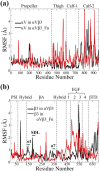
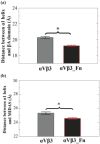
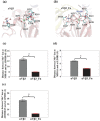
Integrin αVβ3 plays an important role in regulating cellular activities and in human diseases. Although the structure of αVβ3 has been studied by crystallography and electron microscopy, the detailed activation mechanism of integrin αVβ3 induced by fibronectin remains unclear. In this study, we investigated the conformational and dynamical motion changes of Mn2+ -bound integrin αVβ3 by binding to fibronectin with molecular dynamics simulations. Results showed that fibronectin binding to integrin αVβ3 caused the changes of the conformational flexibility of αVβ3 domains, the essential mode of motion for the domains of αV subunit and β3 subunit and the degrees of correlated motion of residues between the domains of αV subunit and β3 subunit of integrin αVβ3. The angle of Propeller domain with respect to the Calf-2 domain of αV subunit and the angle of Hybrid domain with respect to βA domain of β3 subunit significantly increased when integrin αVβ3 was bound to fibronectin. These changes could result in the conformational change tendency of αVβ3 from a bend conformation to an extended conformation and lead to the open swing of Hybrid domain relative to βA domain of β3 subunit, which have demonstrated their importance for αVβ3 activation. Fibronectin binding to integrin αVβ3 significantly decreased the relative position of α1 helix to βA domain and that to metal ion-dependent adhesion site, stabilized Mn2+ ions binding in integrin αVβ3 and changed fibronectin conformation, which are important for αVβ3 activation. Results from this study provide important molecular insight into the "outside-in" activation mechanism of integrin αVβ3 by binding to fibronectin.
Keywords: activation mechanism; conformational and dynamical motion changes; fibronectin; integrin αVβ3; molecular dynamics simulation.
© 2017 The Protein Society.
Figures





Similar articles
-
How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation.J Cell Biol. 2006 Oct 23;175(2):349-60. doi: 10.1083/jcb.200602071. J Cell Biol. 2006. PMID: 17060501 Free PMC article.
-
Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin.Nat Struct Mol Biol. 2014 Apr;21(4):383-8. doi: 10.1038/nsmb.2797. Epub 2014 Mar 23. Nat Struct Mol Biol. 2014. PMID: 24658351 Free PMC article.
-
Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin.J Cell Biol. 2005 Mar 28;168(7):1109-18. doi: 10.1083/jcb.200410068. J Cell Biol. 2005. PMID: 15795319 Free PMC article.
-
High Affinity vs. Native Fibronectin in the Modulation of αvβ3 Integrin Conformational Dynamics: Insights from Computational Analyses and Implications for Molecular Design.PLoS Comput Biol. 2017 Jan 23;13(1):e1005334. doi: 10.1371/journal.pcbi.1005334. eCollection 2017 Jan. PLoS Comput Biol. 2017. PMID: 28114375 Free PMC article.
-
The α-subunit regulates stability of the metal ion at the ligand-associated metal ion-binding site in β3 integrins.J Biol Chem. 2014 Aug 15;289(33):23256-23263. doi: 10.1074/jbc.M114.581470. Epub 2014 Jun 28. J Biol Chem. 2014. PMID: 24975416 Free PMC article.
Cited by
-
Interaction of integrin αvβ3 and fibronectin under fluid shear forces: implications for tumor cell adhesion and migration.Front Cell Dev Biol. 2025 Feb 13;13:1512672. doi: 10.3389/fcell.2025.1512672. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40070879 Free PMC article.
-
Using ELP Repeats as a Scaffold for De Novo Construction of Gadolinium-Binding Domains within Multifunctional Recombinant Proteins for Targeted Delivery of Gadolinium to Tumour Cells.Int J Mol Sci. 2022 Mar 18;23(6):3297. doi: 10.3390/ijms23063297. Int J Mol Sci. 2022. PMID: 35328725 Free PMC article.
-
Fibronectin Regulation of Integrin B1 and SLUG in Circulating Tumor Cells.Cells. 2019 Jun 20;8(6):618. doi: 10.3390/cells8060618. Cells. 2019. PMID: 31226820 Free PMC article.
-
Modeling the structural and dynamical changes of the epithelial calcium channel TRPV5 caused by the A563T variation based on the structure of TRPV6.J Biomol Struct Dyn. 2019 Aug;37(13):3506-3512. doi: 10.1080/07391102.2018.1518790. Epub 2018 Dec 10. J Biomol Struct Dyn. 2019. PMID: 30175942 Free PMC article.
-
Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs).Sci Rep. 2021 Apr 13;11(1):8002. doi: 10.1038/s41598-021-87061-w. Sci Rep. 2021. PMID: 33850196 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

