Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK
- PMID: 28340562
- PMCID: PMC5366108
- DOI: 10.1186/s12879-017-2330-z
Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK
Abstract
Background: We describe drug-induced liver injury (DILI) secondary to antituberculous treatment (ATT) in a large tuberculosis (TB) centre in London; we identify the proportion who had risk factors for DILI and the timing and outcome of DILI.
Methods: We identified consecutive patients who developed DILI whilst on treatment for active TB; patients with active TB without DILI were selected as controls. Comprehensive demographic and clinical data, management and outcome were recorded.
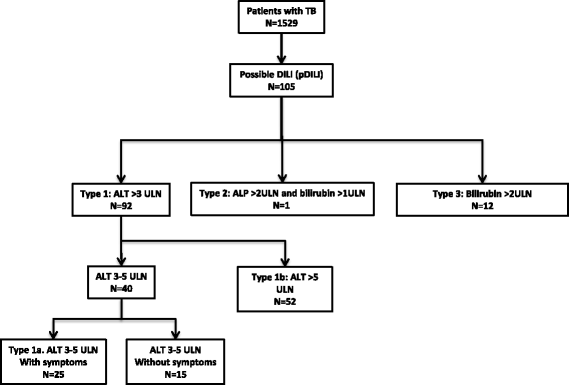
Results: There were 105 (6.9%) cases of ATT-associated DILI amongst 1529 patients diagnosed with active TB between April 2010 and May 2014. Risk factors for DILI were: low patient weight, HIV-1 co-infection, higher baseline ALP, and alcohol intake. Only 25.7% of patients had British or American Thoracic Society defined criteria for liver test (LT) monitoring. Half (53%) of the cases occurred within 2 weeks of starting ATT and 87.6% occurred within 8 weeks. Five (4.8%) of seven deaths were attributable to DILI.
Conclusions: Only a quarter of patients who developed DILI had British or American Thoracic Society defined criteria for pre-emptive LT monitoring, suggesting that all patients on ATT should be considered for universal liver monitoring particularly during the first 8 weeks of treatment.
Keywords: Drug induced liver injury; Hepatotoxicity; Liver failure; Re-introduction regimen; Risk factors; Tuberculosis.
Figures

References
-
- Singanayagam A, Sridhar S, Dhariwal J, Abdel-Aziz D, Munro K, Connell DW, George PM, Molyneaux PL, Cooke GS, Burroughs AK, Lalvani A, Wickremasinghe M, Kon OM. A Comparison between Two Strategies for Monitoring Hepatic Function during Antituberculous Therapy. Am J Respir Crit Care Med. 2012;185(6):653–659. doi: 10.1164/rccm.201105-0850OC. - DOI - PubMed
-
- Yimer G, Gry M, Amogne W, Makonnen E, Habtewold A, Petros Z, Aderaye G, Schuppe-Koistinen I, Lindquist L, Aklillu E. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: a prospective four arm observational study in ethiopian patients. PLoS One. 2014;9(4):e94271. doi: 10.1371/journal.pone.0094271. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

