Lymphocytes have a role in protection, but not in pathogenesis, during La Crosse Virus infection in mice
- PMID: 28340587
- PMCID: PMC5364665
- DOI: 10.1186/s12974-017-0836-3
Lymphocytes have a role in protection, but not in pathogenesis, during La Crosse Virus infection in mice
Abstract
Background: La Crosse Virus (LACV) is a primary cause of pediatric viral encephalitis in the USA and can result in severe clinical outcomes. Almost all cases of LACV encephalitis occur in children 16 years or younger, indicating an age-related susceptibility. This susceptibility is recapitulated in a mouse model where weanling (3 weeks old or younger) mice are susceptible to LACV-induced disease, and adults (greater than 6 weeks) are resistant. Disease in mice and humans is associated with infiltrating leukocytes to the CNS. However, what cell types are infiltrating into the brain during virus infection and how these cells influence pathogenesis remain unknown.
Methods: In the current study, we analyzed lymphocytes recruited to the CNS during LACV-infection in clinical mice, using flow cytometry. We analyzed the contribution of these lymphocytes to LACV pathogenesis in weanling mice using knockout mice or antibody depletion. Additionally, we studied at the potential role of these lymphocytes in preventing LACV neurological disease in resistant adult mice.
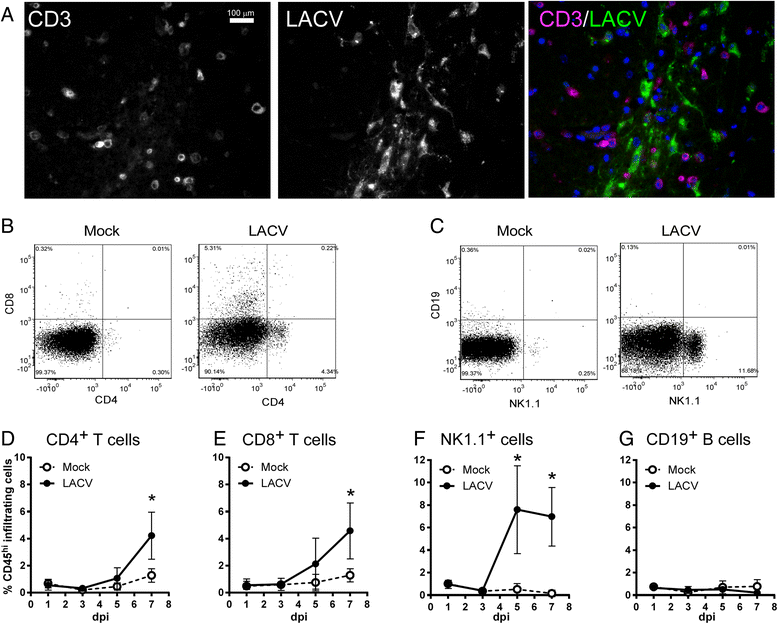
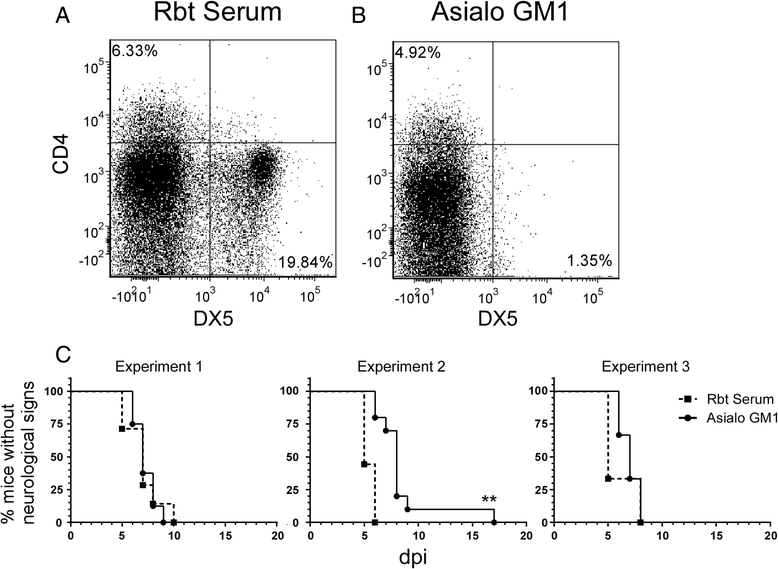
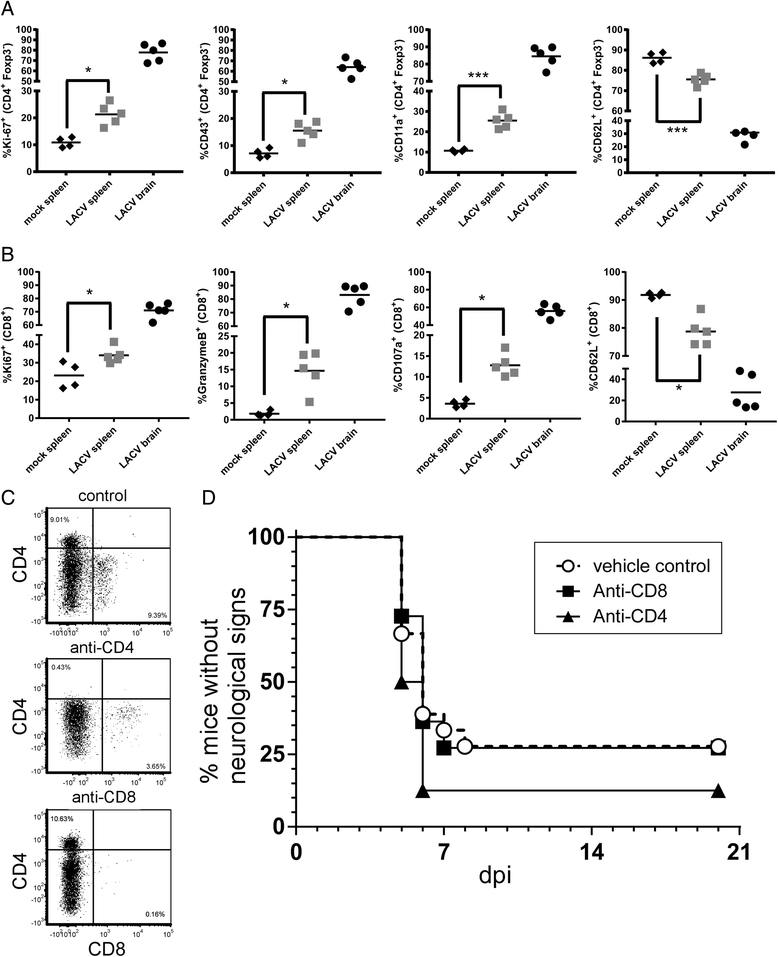
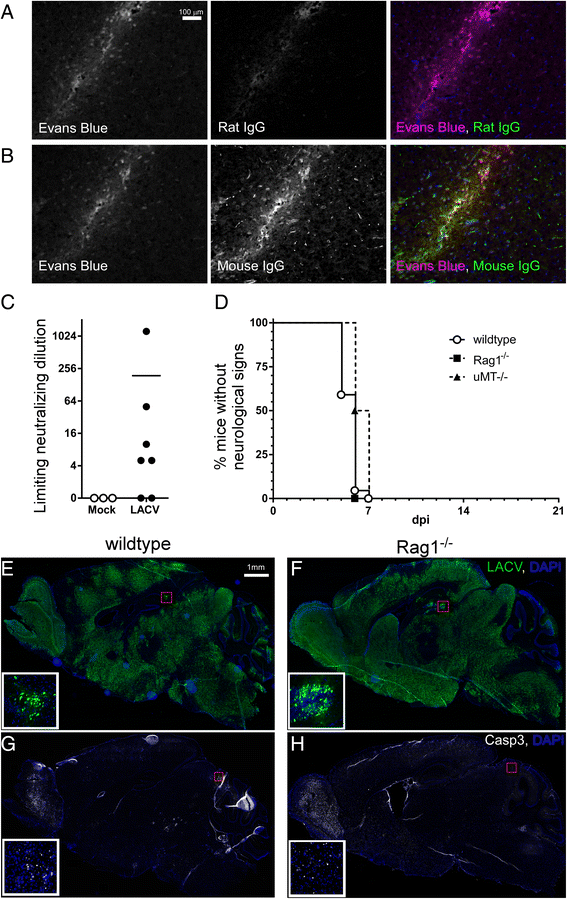
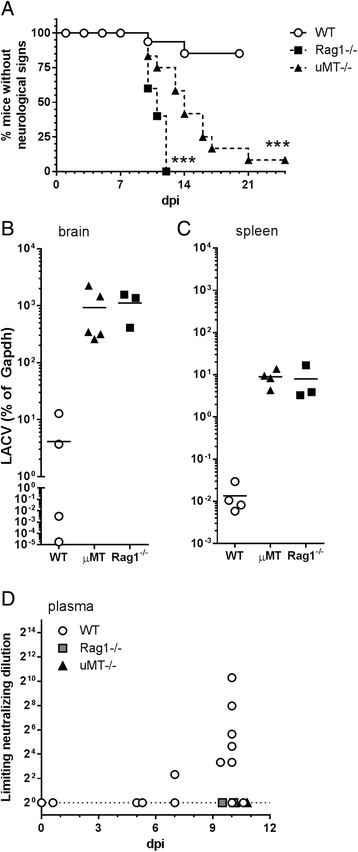
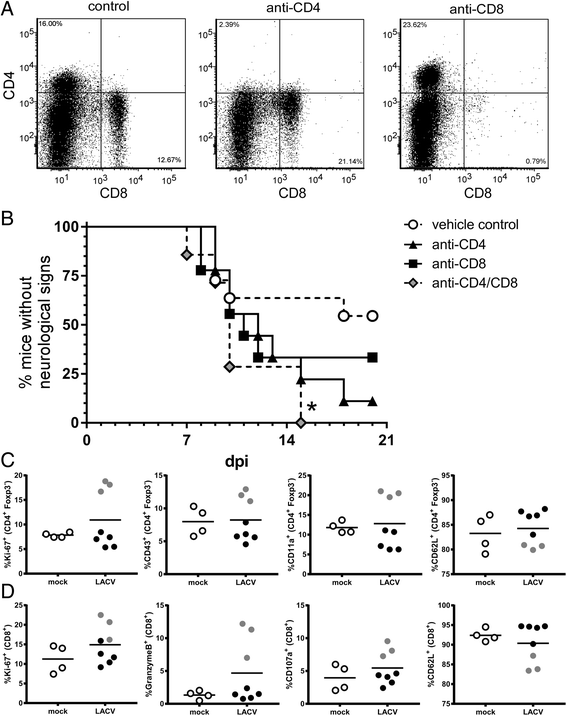
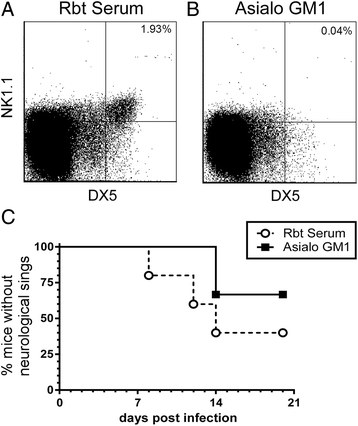
Results: In susceptible weanling mice, disease was associated with infiltrating lymphocytes in the CNS, including NK cells, CD4 T cells, and CD8 T cells. Surprisingly, depletion of these cells did not impact neurological disease, suggesting these cells do not contribute to virus-mediated damage. In contrast, in disease-resistant adult animals, depletion of both CD4 T cells and CD8 T cells or depletion of B cells increased neurological disease, with higher levels of virus in the brain.
Conclusions: Our current results indicate that lymphocytes do not influence neurological disease in young mice, but they have a critical role protecting adult animals from LACV pathogenesis. Although LACV is an acute virus infection, these studies indicate that the innate immune response in adults is not sufficient for protection and that components of the adaptive immune response are necessary to prevent virus from invading the CNS.
Keywords: Brain; Central nervous system; Encephalitis; La Crosse Virus; Lymphocytes.
Figures
Similar articles
-
Age-specific dynamics of neutralizing antibodies, cytokines, and chemokines in response to La Crosse virus infection in mice.J Virol. 2024 Dec 17;98(12):e0176224. doi: 10.1128/jvi.01762-24. Epub 2024 Nov 5. J Virol. 2024. PMID: 39498968 Free PMC article.
-
Age-dependent myeloid dendritic cell responses mediate resistance to la crosse virus-induced neurological disease.J Virol. 2014 Oct;88(19):11070-9. doi: 10.1128/JVI.01866-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008929 Free PMC article.
-
Age influences susceptibility of brain capillary endothelial cells to La Crosse virus infection and cell death.J Neuroinflammation. 2021 Jun 3;18(1):125. doi: 10.1186/s12974-021-02173-4. J Neuroinflammation. 2021. PMID: 34082753 Free PMC article.
-
Innate immune response to La Crosse virus infection.J Neurovirol. 2014 Apr;20(2):150-6. doi: 10.1007/s13365-013-0186-6. Epub 2013 Jul 12. J Neurovirol. 2014. PMID: 23846288 Review.
-
La Crosse Virus: A Comprehensive Review of Its Emerging Public Health Importance.Viral Immunol. 2025 May;38(4):137-147. doi: 10.1089/vim.2024.0088. Epub 2025 Apr 24. Viral Immunol. 2025. PMID: 40274395 Review.
Cited by
-
Identification of age-specific gene regulators of La Crosse virus neuroinvasion and pathogenesis.Nat Commun. 2023 May 18;14(1):2836. doi: 10.1038/s41467-023-37833-x. Nat Commun. 2023. PMID: 37202395 Free PMC article.
-
Pathogenesis and Immune Response of Ebinur Lake Virus: A Newly Identified Orthobunyavirus That Exhibited Strong Virulence in Mice.Front Microbiol. 2021 Feb 1;11:625661. doi: 10.3389/fmicb.2020.625661. eCollection 2020. Front Microbiol. 2021. PMID: 33597934 Free PMC article.
-
Spatiotemporal profile of an optimal host response to virus infection in the primate central nervous system.PLoS Pathog. 2025 Jan 22;21(1):e1012530. doi: 10.1371/journal.ppat.1012530. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39841753 Free PMC article.
-
Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection.Rev Med Virol. 2020 Sep;30(5):e2123. doi: 10.1002/rmv.2123. Epub 2020 Jul 9. Rev Med Virol. 2020. PMID: 32648313 Free PMC article. Review.
-
Throw out the Map: Neuropathogenesis of the Globally Expanding California Serogroup of Orthobunyaviruses.Viruses. 2019 Aug 29;11(9):794. doi: 10.3390/v11090794. Viruses. 2019. PMID: 31470541 Free PMC article. Review.
References
-
- Bennett RS, Gresko AK, Nelson JT, Murphy BR, Whitehead SS. A recombinant chimeric La Crosse virus expressing the surface glycoproteins of Jamestown Canyon virus is immunogenic and protective against challenge with either parental virus in mice or monkeys. J Virol. 2012;86:420–426. doi: 10.1128/JVI.02327-10. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

