Stable high-level expression of factor VIII in Chinese hamster ovary cells in improved elongation factor-1 alpha-based system
- PMID: 28340620
- PMCID: PMC5366130
- DOI: 10.1186/s12896-017-0353-6
Stable high-level expression of factor VIII in Chinese hamster ovary cells in improved elongation factor-1 alpha-based system
Abstract
Background: Recombinant factor VIII (FVIII), used for haemophilia A therapy, is one of the most challenging among the therapeutic proteins produced in heterologous expression systems. Deletion variant of FVIII, in which the entire domain B is replaced by a short linker peptide, was approved for medical use. Efficacy and safety of this FVIII deletion variant are similar to full-length FVIII preparations while the level of production in CHO cells is substantially higher. Typical levels of productivity for CHO cell lines producing deletion variant FVIII-BDD SQ, described elsewhere, are 0.5-2 IU/ml, corresponding to the concentration of FVIII of about 0.2 μg/ml. Using standard vectors based on the cytomegalovirus promoter (CMV) and the dihydrofolate reductase cDNA we have previously obtained the cell line secreting 0.5 IU/ml of FVIII-BDD, which roughly corresponds to the previously published data.
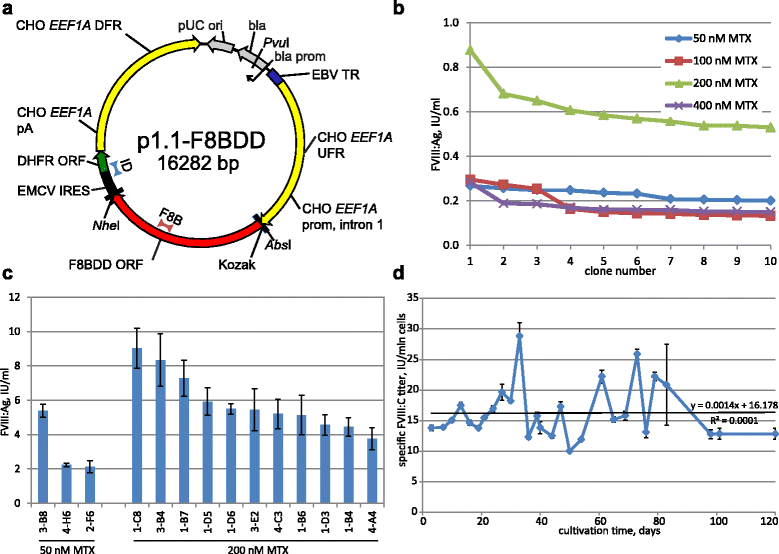
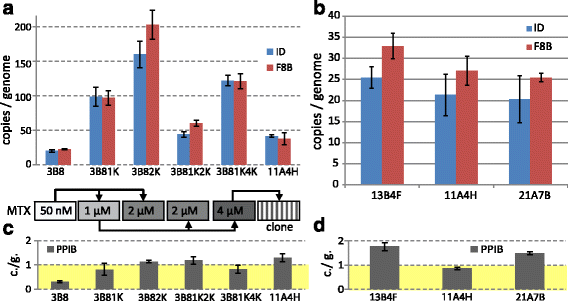
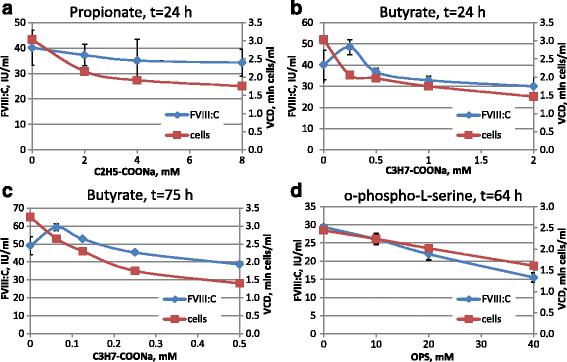
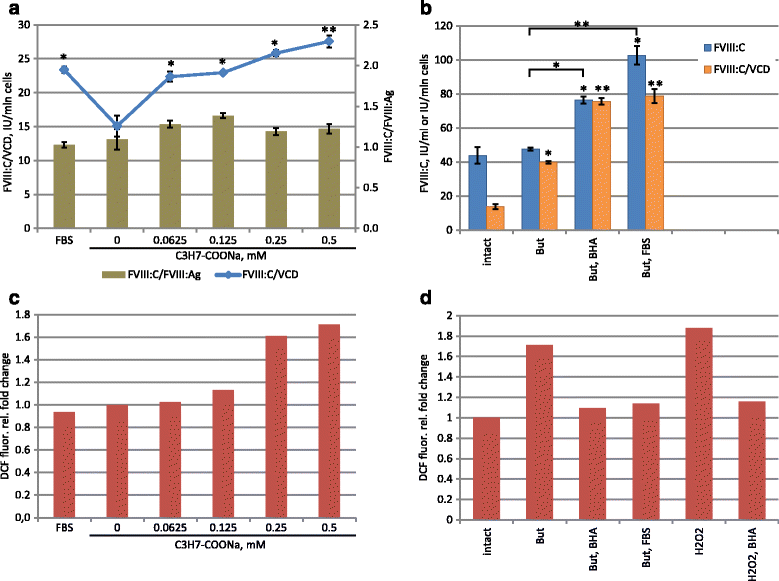
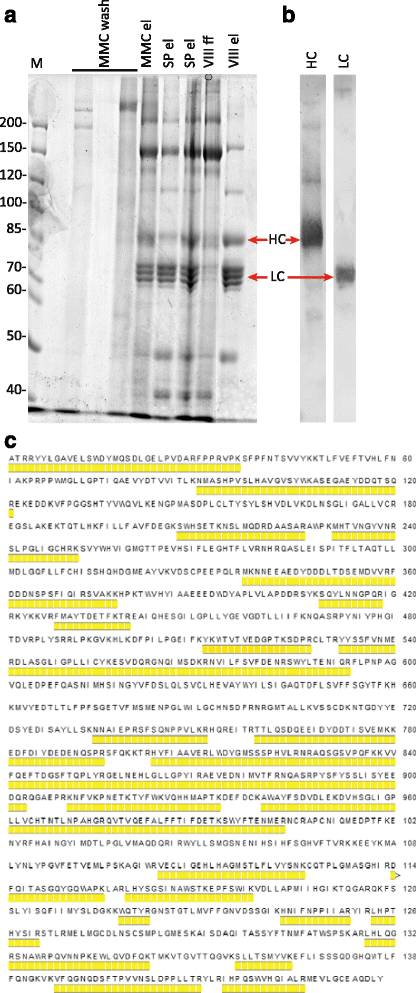
Results: An expression system based on CHO genomic sequences including CHO-EEF1A promoter and Epstein-Barr virus terminal repeat fragment allowed us to achieve 80-fold increase in the production level as compared with the conventional expression system based on the CMV promoter. Immediately after the primary selection FVIII -producing cells secreted 5-10 IU/ml of FVIII-BDD, and after multi-stage methotrexate-driven amplification a stable clonal line 11A4H was selected, secreting 39 IU/ml of FVIII-BDD in the simple batch culturing conditions, which considerably exceeds known indicators for industrial producers of this protein. In contrast to other FVIII-BDD producing lines 11A4H accumulates low proportion of the secreted FVIII on the membrane. Its productivity may be further increased approximately two-fold by adding sodium butyrate and butylated hydroxyanisol to the culture medium. A five-stage purification process for the factor VIII was employed. It allowed isolation of the intact FVIII-BDD as was confirmed by mass spectrometry. Purified FVIII-BDD has a specific activity of 11,000 IU/mg, similar to known recombinant FVIII drugs.
Conclusions: The recombinant FVIII-BDD was produced in CHO cells without addition of any animal-derived materials, purified and characterized. Novel genetic constructions for the expression of heterologous proteins combined with optimized cultivation method allowed to obtain the secretion level of biologically active recombinant FVIII increased by almost ten times as compared with the previously published analogues.
Keywords: CHO cells; Factor VIII; High level expression; Stable cell line generation.
Figures


References
-
- van Dieijen G, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256(7):3433–3442. - PubMed
-
- Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25(2):505–512. doi: 10.1021/bi00350a035. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

