Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8+ T Cells
- PMID: 28341670
- PMCID: PMC5435941
- DOI: 10.4049/jimmunol.1502672
Prime-Boost Immunization Eliminates Metastatic Colorectal Cancer by Producing High-Avidity Effector CD8+ T Cells
Abstract
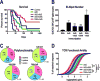
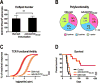
Heterologous prime-boost immunization with plasmid DNA and viral vector vaccines is an emerging approach to elicit CD8+ T cell-mediated immunity targeting pathogens and tumor Ags that is superior to either monotherapy. Yet, the mechanisms underlying the synergy of prime-boost strategies remain incompletely defined. In this study, we examine a DNA and adenovirus (Ad5) combination regimen targeting guanylyl cyclase C (GUCY2C), a receptor expressed by intestinal mucosa and universally expressed by metastatic colorectal cancer. DNA immunization efficacy was optimized by i.m. delivery via electroporation, yet it remained modest compared with Ad5. Sequential immunization with DNA and Ad5 produced superior antitumor efficacy associated with increased TCR avidity, whereas targeted disruption of TCR avidity enhancement eliminated GUCY2C-specific antitumor efficacy, without affecting responding T cell number or cytokine profile. Indeed, functional TCR avidity of responding GUCY2C-specific CD8+ T cells induced by various prime or prime-boost regimens correlated with antitumor efficacy, whereas T cell number and cytokine profile were not. Importantly, although sequential immunization with DNA and Ad5 maximized antitumor efficacy through TCR avidity enhancement, it produced no autoimmunity, reflecting sequestration of GUCY2C to intestinal apical membranes and segregation of mucosal and systemic immunity. Together, TCR avidity enhancement may be leveraged by prime-boost immunization to improve GUCY2C-targeted colorectal cancer immunotherapeutic efficacy and patient outcomes without concomitant autoimmune toxicity.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures

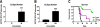
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. - PubMed
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, I.S. Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. - PubMed
-
- Appaiahgari MB, Vrati S. Clinical development of IMOJEV (R)–a recombinant Japanese encephalitis chimeric vaccine (JE-CV) Expert Opin Biol Ther. 2012;12:1251–1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

