Parkinson's disease-associated receptor GPR37 is an ER chaperone for LRP6
- PMID: 28341812
- PMCID: PMC5412897
- DOI: 10.15252/embr.201643585
Parkinson's disease-associated receptor GPR37 is an ER chaperone for LRP6
Abstract
Wnt/β-catenin signaling plays a key role in embryonic development, stem cell biology, and neurogenesis. However, the mechanisms of Wnt signal transmission, notably how the receptors are regulated, remain incompletely understood. Here we describe that the Parkinson's disease-associated receptor GPR37 functions in the maturation of the N-terminal bulky β-propellers of the Wnt co-receptor LRP6. GPR37 is required for Wnt/β-catenin signaling and protects LRP6 from ER-associated degradation via CHIP (carboxyl terminus of Hsc70-interacting protein) and the ATPase VCP GPR37 is highly expressed in neural progenitor cells (NPCs) where it is required for Wnt-dependent neurogenesis. We conclude that GPR37 is crucial for cellular protein quality control during Wnt signaling.
Keywords: ER‐associated degradation; GPR37; LRP6; PAEL‐R; Wnt signaling.
© 2017 The Authors.
Figures
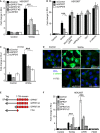
- A, B
Topflash reporter assay in HEK293T cells upon knockdown of GPR37 using siRNA pool or single siRNAs, or siLRP5/6. Cells were stimulated with Wnt3a‐conditioned media or by transfection with the indicated constructs (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- C
Topflash reporter assay in H1703 cells upon knockdown of GPR37 or LRP5/6. Cells were treated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- D
Immunofluorescence microscopy showing β‐catenin accumulation (green) in H1703 cells. Cells were transfected with the indicated siRNAs and stimulated with Wnt3a‐conditioned media for 3 h. Scale bar: 10 μm.
- E
GPR37 deletion constructs used in this study. All deletion constructs contain the Kremen signal peptide (SP) followed by an N‐terminal tag. The transmembrane region (TM) is shown in red.
- F
Topflash reporter assay in HEK293T cells upon overexpression of GPR37 deletion constructs shown in (E) in combination with the indicated constructs, and stimulated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test). Note that the lack of activity in GPR37ΔC may be possibly due to misfolding of the seven transmembrane domains.
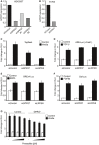
- A, B
qPCR representing the knockdown efficiencies of the indicated single siRNAs or siRNA pool (siGPR37) used in Fig 1.
- C–F
Reporter assays for Wnt (C), TGFβ (D), BMP4 (E), or FGF (F) signaling in HEK293T cells upon knockdown of GPR37 or LRP5/6. Cells were transfected as described in the Materials and Methods section, and stimulated with conditioned media (C) or the indicated recombinant proteins (D–F) (mean values ± SD, n = 3).
- G
Topflash reporter assay in HEK293T cells transfected with either control vector or GPR37. Cells were treated for 24 h with recombinant prosaptide (0–3 μM) in the presence or absence of Wnt3a‐conditioned media (mean values ± SD, n = 3).

Topflash reporter assay in HEK293T cells upon siRNA knockdown of GPR37. Cells were transfected with control vector or GPR37‐1TM to rescue the siGPR37 effect, and treated as indicated (mean values ± SD, n = 3).
Immunoblot of transfected V5‐GPR37 and V5‐GPR37‐1TM in HEK293T cells. Shown is a representative experiment performed five times.

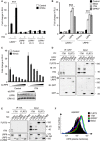
- A
Topflash reporter assay in LRP6 +/+ and LRP6 −/− HEK293T cells. Cells were transfected with GPR37‐1TM (1TM) or control vector and treated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- B
Co‐immunoprecipitation of overexpressed LRP6 and the indicated GPR37 constructs. Immunoblots of immunoprecipitates from HEK293T cells transfected with the indicated FLAG‐ and V5‐tagged constructs. IgG bands are indicated with an asterisk. Note that LRP6 binds preferentially the lower molecular weight band of GPR37‐1TM, which represents the ER form of GPR37‐1TM. Shown is a representative experiment carried out three times.
- C
Co‐immunoprecipitation of endogenous LRP6. Immunoblots of immunoprecipitates from HEK293T cells transfected with the indicated V5‐tagged constructs. GSK3 serves as a negative control. Shown is a representative experiment carried out five times.
- D, E
Immunoblots of H1703 (D) or HEK293T (E) cell lysates upon knockdown of GPR37. Transferrin receptor serves as a loading control. Shown are representative experiments carried out eight and seven times, respectively.
- F, G
FACS analyses of cell surface LRP6 protein levels upon knockdown of GPR37 or LRP6 in H1703 (F) or HEK293T (G) cells. Blue dotted line shows background signal upon staining with control IgGs. Shown are representative experiments carried out three times.
- H
qPCR analysis of LRP6 expression levels in HEK293T or H1703 cells upon knockdown of GPR37 (mean values ± SD, n = 3).
- A, B
Topflash reporter assays in LRP6+/+ HEK293T cells and three individual LRP6−/− HEK293T clones. Cells were transfected with the indicated constructs and treated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- C
Upper panel, Topflash reporter assays in HEK293T cells upon dose‐dependent siRNA knockdown of LRP6 (0–100 nM siRNA) and transfection with GPR37‐1TM. Cells were treated as indicated (SD, n = 3). Lower panel, immunoblots show the knockdown efficiencies of LRP6 in the Topflash assay.
- D, E
Co‐immunoprecipitation of GPR37‐1TM with LRP5 (D) and LRP3 (E). FLRT3 and LILRB2 transmembrane proteins serve as controls. Note that in (E) LRP6 but not LRP3 co‐immunoprecipitates GPR37‐1TM. Shown is a representative experiment carried out three times.
- F
FACS analysis of LRP6 cell surface levels upon transfection with the indicated constructs. Shown is a representative experiment carried out three times.
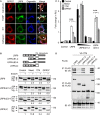
Immunofluorescence microscopy of HEK293T cells transfected with V5‐GPR37 and HA‐LRP6 together with the organelle markers mCherry‐calreticulin (ER), mCherry‐TGNP (Golgi), mCherry‐SiT (trans‐Golgi), or mCherry‐cathepsin (lysosome), which are shown in blue. White signal in merge shows co‐localization of all three labels (arrows). Scale bar: 10 μm.
Representative immunoblots of HEK293T cells transfected with the indicated LRP6 deletion constructs and co‐transfected with control vector, Mesd, GPR37‐1TM, or GPR37. Cell lysates were subjected to EndoH treatment as indicated. Ma, mature LRP6; im, immature; dg, deglycosylated. Numbers under lanes indicate the ratio of mature (ma) to immature (im) LRP6 band from densitometric analysis. Shown are representative experiments that were carried out three times.
Topflash reporter assay in HEK293T cells transfected with the indicated LRP6 constructs and co‐transfected with control vector, Mesd, GPR37‐1TM, or GPR37. Cells were treated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
Co‐immunoprecipitation of GPR37‐1TM with LRP6 deletion constructs. Immunoblots of immunoprecipitates from HEK293T cells transfected with the indicated constructs. The asterisk indicates an unspecific band. Shown are representative experiments that were performed six times.

Immunofluorescence microscopy of transfected LRP6 and V5‐GPR37‐1TM with the organelle markers calreticulin (ER), TGNP (Golgi), SiT (trans‐Golgi), or cathepsin (lysosomes), all shown in blue. White signal in merge shows co‐localization of all three constructs (arrows). Scale bar: 10 μm.
Immunoblots of immunoprecipitated GPR37‐1TM with LRP6. Note that GPR37 only binds the immature (im, ER) form of LRP6, but not to the mature (ma) form. Shown is a representative experiment carried out three times.
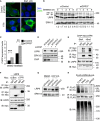
- A
Immunofluorescence microscopy of HEK293T cells transfected with HA‐LRP6 (green) upon co‐transfection with V5‐GPR37‐1TM or V5‐FLRT3 (Control), and treated with brefeldin A (Bref.) or vehicle (−) for 4 h as indicated. Scale bar: 10 μm.
- B
Representative immunoblots of endogenous LRP6 from H1703 cells upon knockdown of GPR37 and treatment with brefeldin A as indicated. Values under the immunoblots show the quantitation of immature, low molecular weight LRP6, normalized to the loading control ERK1/2. Asterisk indicates an unspecific band. Shown are representative experiments that were carried out three times.
- C
qPCR analysis of the ER stress marker CHOP upon knockdown of GPR37 and treatment with the ER stress‐inducing agent brefeldin A for 6 h (mean values ± SD, n = 3; **P < 0.01, ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- D
Immunoblots of H1703 cells transfected with the indicated siRNAs. ERK1/2 serves as a loading control. Shown are representative experiments that were performed three times.
- E, F
Immunoblots of immunoprecipitates from HEK293T cells transfected with the indicated constructs. Cells were treated with brefeldin A (E and F) or vehicle for 4 h (E). Shown are representative experiments that were performed five times.
- G
Immunoblots of H1703 cells upon knockdown of GPR37 and treatment with the VCP inhibitor NMS‐873, or the vehicle DMSO, for 5 h. ERK1/2 serves as a loading control. Shown are representative experiments that were performed three times.
- H
LRP6 ubiquitination analysis. Representative immunoblots of immunoprecipitated FLAG‐LRP6 in HEK293T cells transfected as indicated and treated with the proteasomal inhibitor MG‐132 and the lysosomal inhibitor chloroquine for 6 h to induce accumulation of ubiquitinated LRP6 (LRP6‐Ubn). Ub‐HA, ubiquitin‐HA. Shown are representative experiments that were performed five times.
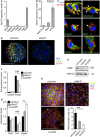
- A, B
qPCR expression analysis of Gpr37 in the indicated mouse tissues or isolated NPCs. Gpr37 expression was normalized against Gapdh (mean values ± SD, n = 3; one‐way ANOVA followed by Holm–Sidak test).
- C
Immunofluorescence microscopy of endogenous GPR37 (red) with LRP6, calreticulin (ER), TGN38 (Golgi), EEA1 (early endosome), clathrin (endocytic vesicles), or pan‐cadherin (cell junctions), shown in green, in NPCs from disassociated neurospheres. Yellow signal shows co‐localization (arrows). Scale bar: 10 μm.
- D
Immunofluorescence microscopy of endogenous LRP6 (green) and GPR37 (red) in neurospheres upon siGpr37 treatment. DNA (Hoechst) is shown in blue. Scale bar: 100 μm.
- E
Immunoblot analysis of LRP6 protein levels in NPC cells upon siRNA knockdown of GPR37 or LRP5/6. Note that siGpr37 reduces LRP6 protein levels, without affecting its mRNA expression (see Fig EV5D). Shown are representative experiments that were carried out three times.
- F, G
qPCR expression analysis of the Wnt target gene Sp5 (F) and the indicated neural fate markers (G) in NPCs upon siRNA knockdown of GPR37 or LRP5/6. Neurospheres were treated for 5 h with control or Wnt3a‐conditioned media. Expression was normalized against Hprt (mean values ± SE, n = 5; *P < 0.05, ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
- H
Immunofluorescence microscopy of MAP2 (green) and NG2 (red) in differentiating NPCs. Neurospheres were treated with the indicated siRNA and cultured in neuronal differentiation medium (see Materials and Methods). Bottom right, percentage of MAP2‐positive cells per colony (mean values ± SE, n = 5; **P < 0.01, one‐way ANOVA followed by Holm–Sidak test). Scale bar: 100 μm.
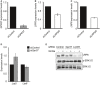
- A–D
qPCR showing the knockdown efficiencies of GPR37 (A), LRP5 (B), LRP6 (C), or LRP5 and LRP6 mRNA levels in NPCs upon knockdown of GPR37 (D) (mean values ± SE, n = 5).
- E
Immunoblots showing phospho‐ERK1/2 levels in NPCs upon siRNA knockdown of GPR37 or LRP5/6. Cells were treated with control or Wnt3a‐conditioned media for 5 h. Shown is a representative experiment performed three times.
References
-
- Klaus A, Birchmeier W (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8: 387–398 - PubMed
-
- Clevers H, Nusse R (2012) Wnt/beta‐catenin signaling and disease. Cell 149: 1192–1205 - PubMed
-
- Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13: 11–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

