Embryonic defence mechanisms against glucose-dependent oxidative stress require enhanced expression of Alx3 to prevent malformations during diabetic pregnancy
- PMID: 28341857
- PMCID: PMC5428206
- DOI: 10.1038/s41598-017-00334-1
Embryonic defence mechanisms against glucose-dependent oxidative stress require enhanced expression of Alx3 to prevent malformations during diabetic pregnancy
Abstract
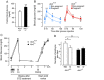
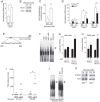
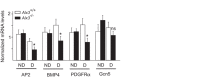
Oxidative stress constitutes a major cause for increased risk of congenital malformations associated to severe hyperglycaemia during pregnancy. Mutations in the gene encoding the transcription factor ALX3 cause congenital craniofacial and neural tube defects. Since oxidative stress and lack of ALX3 favour excessive embryonic apoptosis, we investigated whether ALX3-deficiency further increases the risk of embryonic damage during gestational hyperglycaemia in mice. We found that congenital malformations associated to ALX3-deficiency are enhanced in diabetic pregnancies. Increased expression of genes encoding oxidative stress-scavenging enzymes in embryos from diabetic mothers was blunted in the absence of ALX3, leading to increased oxidative stress. Levels of ALX3 increased in response to glucose, but ALX3 did not activate oxidative stress defence genes directly. Instead, ALX3 stimulated the transcription of Foxo1, a master regulator of oxidative stress-scavenging genes, by binding to a newly identified binding site located in the Foxo1 promoter. Our data identify ALX3 as an important component of the defence mechanisms against the occurrence of developmental malformations during diabetic gestations, stimulating the expression of oxidative stress-scavenging genes in a glucose-dependent manner via Foxo1 activation. Thus, ALX3 deficiency provides a novel molecular mechanism for developmental defects arising from maternal hyperglycaemia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

