Use of BRCA Mutation Test in the U.S., 2004-2014
- PMID: 28342662
- PMCID: PMC5370584
- DOI: 10.1016/j.amepre.2017.01.027
Use of BRCA Mutation Test in the U.S., 2004-2014
Abstract
Introduction: BRCA mutation testing has been used for screening women at high risk of breast and ovarian cancer and for selecting the best treatment for those with breast cancer. To optimize the infrastructure and medical resources allocation for genetic testing, it is important to understand the use of BRCA mutation testing in the U.S. health system.
Methods: This retrospective cohort study included 53,254 adult women with insurance claims for BRCA mutation testing between 2004 and 2014 from ClinformaticsTM Data Mart Database. Data analysis was performed in 2016. This study assessed trends in the use of BRCA mutation testing in women with previously diagnosed breast or ovarian cancer and those without (unaffected women).
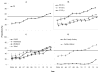
Results: Between 2004 and 2014, of those receiving BRCA testing, the proportion of BRCA tests performed in unaffected women increased significantly (p<0.001), from 24.3% in 2004 to 61.5% in 2014. An increase in the proportion of BRCA tests used in unaffected women was found in each characteristic subgroup. In 2014, most subgroups had a proportion surpassing 50%, except for those aged 51-65 years and those without a family history of breast cancer. There was a much lower proportion of those aged 20-40 years among tested women with previously diagnosed breast or ovarian cancer than in unaffected women (17.6% vs 41.7%, p<0.001).
Conclusions: During the past decade, the role of BRCA testing has gradually shifted from being used primarily in cancer patients to being used in unaffected women in the U.S.
Copyright © 2017 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Increased Use of BRCA Mutation Test in Unaffected Women Over the Period 2004-2014 in the U.S.: Further Evidence of the "Angelina Jolie Effect"?Am J Prev Med. 2017 Nov;53(5):e195-e196. doi: 10.1016/j.amepre.2017.05.016. Am J Prev Med. 2017. PMID: 29054246 No abstract available.
-
Authors' Response: "Angelina Jolie Effect" on the Shifting Role of BRCA Testing in the U.S.Am J Prev Med. 2017 Nov;53(5):e197-e199. doi: 10.1016/j.amepre.2017.05.015. Am J Prev Med. 2017. PMID: 29054247 No abstract available.
References
-
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. https://doi.org/10.1126/science.7545954. - DOI - PubMed
-
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. https://doi.org/10.1038/378789a0. - DOI - PubMed
-
- FitzGerald MG, MacDonald DJ, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with early-onset breast cancer. N Engl J Med. 1996;334(3):143–149. https://doi.org/10.1056/NEJM199601183340302. - DOI - PubMed
-
- Narod SA. BRCA mutations in the management of breast cancer: the state of the art. Nat Rev Clin Oncol. 2010;7(12):702–707. https://doi.org/10.1038/nrclinonc.2010.166. - DOI - PubMed
-
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. https://doi.org/10.1200/JCO.2011.39.8545. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

