Evidence that increased Kcnj6 gene dose is necessary for deficits in behavior and dentate gyrus synaptic plasticity in the Ts65Dn mouse model of Down syndrome
- PMID: 28342823
- PMCID: PMC5446050
- DOI: 10.1016/j.nbd.2017.03.009
Evidence that increased Kcnj6 gene dose is necessary for deficits in behavior and dentate gyrus synaptic plasticity in the Ts65Dn mouse model of Down syndrome
Abstract
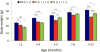
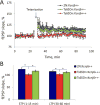
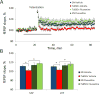
Down syndrome (DS), trisomy 21, is caused by increased dose of genes present on human chromosome 21 (HSA21). The gene-dose hypothesis argues that a change in the dose of individual genes or regulatory sequences on HSA21 is necessary for creating DS-related phenotypes, including cognitive impairment. We focused on a possible role for Kcnj6, the gene encoding Kir3.2 (Girk2) subunits of a G-protein-coupled inwardly-rectifying potassium channel. This gene resides on a segment of mouse Chromosome 16 that is present in one extra copy in the genome of the Ts65Dn mouse, a well-studied genetic model of DS. Kir3.2 subunit-containing potassium channels serve as effectors for a number of postsynaptic metabotropic receptors including GABAB receptors. Several studies raise the possibility that increased Kcnj6 dose contributes to synaptic and cognitive abnormalities in DS. To assess directly a role for Kcnj6 gene dose in cognitive deficits in DS, we produced Ts65Dn mice that harbor only 2 copies of Kcnj6 (Ts65Dn:Kcnj6++- mice). The reduction in Kcnj6 gene dose restored to normal the hippocampal level of Kir3.2. Long-term memory, examined in the novel object recognition test with the retention period of 24h, was improved to the level observed in the normosomic littermate control mice (2N:Kcnj6++). Significantly, both short-term and long-term potentiation (STP and LTP) was improved to control levels in the dentate gyrus (DG) of the Ts65Dn:Kcnj6++- mouse. In view of the ability of fluoxetine to suppress Kir3.2 channels, we asked if fluoxetine-treated DG slices of Ts65Dn:Kcnj6+++ mice would rescue synaptic plasticity. Fluoxetine increased STP and LTP to control levels. These results are evidence that increased Kcnj6 gene dose is necessary for synaptic and cognitive dysfunction in the Ts65Dn mouse model of DS. Strategies aimed at pharmacologically reducing channel function should be explored for enhancing cognition in DS.
Keywords: Cognition; Down syndrome critical region; Fluoxetine; Genotype-phenotype relationship; Kcnj6; Kir3.2; Learning; Locomotor activity; Long-term potentiation; Mouse models; Novel object recognition; Synaptic plasticity; Ts65Dn; Y-maze.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
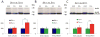

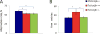
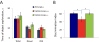
Similar articles
-
Neuroanatomical alterations and synaptic plasticity impairment in the perirhinal cortex of the Ts65Dn mouse model of Down syndrome.Neurobiol Dis. 2017 Oct;106:89-100. doi: 10.1016/j.nbd.2017.06.017. Epub 2017 Jun 23. Neurobiol Dis. 2017. PMID: 28651891
-
Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome.Neurobiol Dis. 2012 Feb;45(2):683-91. doi: 10.1016/j.nbd.2011.10.009. Epub 2011 Oct 17. Neurobiol Dis. 2012. PMID: 22062771 Free PMC article.
-
Infantile spasms in down syndrome: Rescue by knockdown of the GIRK2 channel.Ann Neurol. 2016 Oct;80(4):511-21. doi: 10.1002/ana.24749. Epub 2016 Aug 13. Ann Neurol. 2016. PMID: 27462820
-
Mouse models of Down syndrome: gene content and consequences.Mamm Genome. 2016 Dec;27(11-12):538-555. doi: 10.1007/s00335-016-9661-8. Epub 2016 Aug 18. Mamm Genome. 2016. PMID: 27538963 Free PMC article. Review.
-
On the cause of mental retardation in Down syndrome: extrapolation from full and segmental trisomy 16 mouse models.Brain Res Brain Res Rev. 2001 Apr;35(2):115-45. doi: 10.1016/s0926-6410(00)00074-4. Brain Res Brain Res Rev. 2001. PMID: 11336779 Review.
Cited by
-
Repeated Methylglyoxal Treatment Depletes Dopamine in the Prefrontal Cortex, and Causes Memory Impairment and Depressive-Like Behavior in Mice.Neurochem Res. 2020 Feb;45(2):354-370. doi: 10.1007/s11064-019-02921-2. Epub 2019 Nov 30. Neurochem Res. 2020. PMID: 31786717
-
Prenatal and Postnatal Pharmacotherapy in Down Syndrome: The Search to Prevent or Ameliorate Neurodevelopmental and Neurodegenerative Disorders.Annu Rev Pharmacol Toxicol. 2022 Jan 6;62:211-233. doi: 10.1146/annurev-pharmtox-041521-103641. Annu Rev Pharmacol Toxicol. 2022. PMID: 34990205 Free PMC article.
-
GABAB Receptors and Cognitive Processing in Health and Disease.Curr Top Behav Neurosci. 2022;52:291-329. doi: 10.1007/7854_2021_231. Curr Top Behav Neurosci. 2022. PMID: 34382179 Review.
-
Nuclear Reorganization in Hippocampal Granule Cell Neurons from a Mouse Model of Down Syndrome: Changes in Chromatin Configuration, Nucleoli and Cajal Bodies.Int J Mol Sci. 2021 Jan 27;22(3):1259. doi: 10.3390/ijms22031259. Int J Mol Sci. 2021. PMID: 33514010 Free PMC article.
-
Enhanced GIRK2 channel signaling in Down syndrome: A feasible role in the development of abnormal nascent neural circuits.Front Genet. 2022 Sep 12;13:1006068. doi: 10.3389/fgene.2022.1006068. eCollection 2022. Front Genet. 2022. PMID: 36171878 Free PMC article. Review.
References
-
- Altafaj X, et al. Normalization of Dyrk1A expression by AAV2/1-shDyrk1A attenuates hippocampal-dependent defects in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2013;52:117–27. - PubMed
-
- Baddeley A, Jarrold C. Working memory and Down syndrome. J Intellect Disabil Res. 2007;51:925–31. - PubMed
-
- Basel-Vanagaite L, et al. Keppen-Lubinsky syndrome: Expanding the phenotype. Am J Med Genet A. 2009;149A:1827–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

