Heteromeric complexes of aldo-keto reductase auxiliary KVβ subunits (AKR6A) regulate sarcolemmal localization of KV1.5 in coronary arterial myocytes
- PMID: 28342889
- PMCID: PMC5610061
- DOI: 10.1016/j.cbi.2017.03.011
Heteromeric complexes of aldo-keto reductase auxiliary KVβ subunits (AKR6A) regulate sarcolemmal localization of KV1.5 in coronary arterial myocytes
Abstract
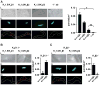
Redox-sensitive potassium channels consisting of the voltage-gated K+ (KV) channel pore subunit KV1.5 regulate resting membrane potential and thereby contractility of vascular smooth muscle cells. Members of the KV1 family associate with cytosolic auxiliary β subunits, which are members of the aldo-keto reductase (AKR) superfamily (AKR6A subfamily). The Kvβ subunits have been proposed to regulate Kv1 gating via pyridine nucleotide cofactor binding. However, the molecular identity of KVβ subunits that associate with native KV1.5 channels in the vasculature is unknown. Here, we examined mRNA and protein expression of KVβ subunits and tested whether KVβ isoforms interact with KV1.5 channels in murine coronary arteries. We detected KVβ1 (AKR6A3), KVβ2 (AKR6A5) and KVβ3 (AKR6A9) transcripts and KVβ1 and KVβ2 protein in left anterior descending coronary arteries by real time quantitative PCR and Western blot, respectively. In situ proximity ligation assays indicated abundant protein-protein interactions between KV1.5/KVβ1, KV1.5/KVβ2 and KVβ1/β2 in coronary arterial myocytes. Confocal microscopy and membrane fractionation analyses suggest that arterial myocytes from KVβ2-null mice have reduced abundance of sarcolemmal KV1.5. Together, data suggest that in coronary arterial myocytes, KV1.5 channels predominantly associate with KVβ1 and KVβ2 proteins and that KVβ2 performs a chaperone function for KV1.5 channels in arterial myocytes, thereby facilitating Kv1α trafficking and membrane localization.
Keywords: Myocardial blood flow; Nicotinamide adenine dinucleotide; Oxidoreductase; Potassium channels; Vascular smooth muscle.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


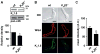
References
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
-
- Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvbeta subunits increase expression of Kv4.3 channels by interacting with their C termini. The Journal of biological chemistry. 2001;276:4839–4844. - PubMed
-
- Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS letters. 2002;528:183–188. - PubMed
-
- Gulbis JM, Zhou M, Mann S, MacKinnon R. Structure of the cytoplasmic beta subunit-T1 assembly of voltage-dependent K+ channels. Science. 2000;289:123–127. - PubMed
-
- Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev. 2010;90:755–796. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

