Focal Brain Injury Associated with a Model of Severe Hypoxic-Ischemic Encephalopathy in Nonhuman Primates
- PMID: 28343228
- PMCID: PMC5519439
- DOI: 10.1159/000456658
Focal Brain Injury Associated with a Model of Severe Hypoxic-Ischemic Encephalopathy in Nonhuman Primates
Abstract
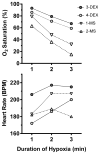
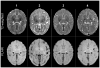
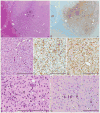
Worldwide, hypoxic-ischemic encephalopathy (HIE) is a major cause of neonatal mortality and morbidity. To better understand the mechanisms contributing to brain injury and improve outcomes in neonates with HIE, better preclinical animal models that mimic the clinical situation following birth asphyxia in term newborns are needed. In an effort to achieve this goal, we modified our nonhuman primate model of HIE induced by in utero umbilical cord occlusion (UCO) to include postnatal hypoxic episodes, in order to simulate apneic events in human neonates with HIE. We describe a cohort of 4 near-term fetal Macaca nemestrina that underwent 18 min of in utero UCO, followed by cesarean section delivery, resuscitation, and subsequent postnatal mechanical ventilation, with exposure to intermittent daily hypoxia (3 min, 8% O2 3-8 times daily for 3 days). After delivery, all animals demonstrated severe metabolic acidosis (pH 7 ± 0.12; mean ± SD) and low APGAR scores (<5 at 10 min of age). Three of 4 animals had both electrographic and clinical seizures. Serial blood samples were collected and plasma metabolites were determined by 2-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC × GC-TOFMS). The 4 UCO animals and a single nonasphyxiated animal (delivered by cesarean section but without exposure to UCO or prolonged sedation) underwent brain magnetic resonance imaging (MRI) on day 8 of life. Thalamic injury was present on MRI in 3 UCO animals, but not in the control animal. Following necropsy on day 8, brain histopathology revealed neuronal injury/loss and gliosis in portions of the ventrolateral thalamus in all 4 UCO, with 2 animals also demonstrating putamen/globus pallidus involvement. In addition, all 4 UCO animals demonstrated brain stem gliosis, with neuronal loss present in the midbrain, pons, and lateral medulla in 3 of 4 animals. Transmission electron microscopy imaging of the brain tissues was performed, which demonstrated ultrastructural white matter abnormalities, characterized by perinuclear vacuolation and axonal dilation, in 3 of 4 animals. Immunolabeling of Nogo-A, a negative regulator of neuronal growth, was not increased in the injured brains compared to 2 control animals. Using GC × GC-TOFMS, we identified metabolites previously recognized as potential biomarkers of perinatal asphyxia. The basal ganglia-thalamus-brain stem injury produced by UCO is consistent with the deep nuclear/brainstem injury pattern seen in human neonates after severe, abrupt hypoxic-ischemic insults. The UCO model permits timely detection of biomarkers associated with specific patterns of neonatal brain injury, and it may ultimately be useful for validating therapeutic strategies to treat neonatal HIE.
Keywords: Dexmedetomidine; Hypoxic-ischemic encephalopathy; Magnetic resonance imaging; Perinatal asphyxia.
© 2017 S. Karger AG, Basel.
Figures





References
-
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE Child Health Epidemiology Reference Group of WHO Unicef. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. - PubMed
-
- Executive summary. Neonatal encephalopathy and neurologic outcome. American College of Obstetricians and Gynecologists. Obstetrics & Gynecology. 2014;123:896–901. - PubMed
-
- Takenouchi T, Kasdorf E, Engel M, Grunebaum A, Perlman JM. Changing pattern of perinatal brain injury in term infants in recent years. Pediatr Neurol. 2012;46:106–110. - PubMed
-
- Juul SE, Aylward E, Richards T, McPherson RJ, Kuratani J, Burbacher TM. Prenatal cord clamping in newborn Macaca nemestrina: a model of perinatal asphyxia. Dev Neurosci. 2007;29:311–320. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child H, Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

