Muscle Injury After Intramuscular Administration of Diclofenac: A Case Report Supported by Magnetic Resonance Imaging
- PMID: 28343290
- PMCID: PMC5366168
- DOI: 10.1007/s40800-017-0049-9
Muscle Injury After Intramuscular Administration of Diclofenac: A Case Report Supported by Magnetic Resonance Imaging
Abstract
Intramuscular injection of diclofenac is still frequently practiced, although there is ample evidence that the risk of local tissue intolerability is highly underestimated. The aim of this study was to evaluate local toxicity in a patient using magnetic resonance imaging. A patient who gave written informed consent received a medically indicated intramuscular administration of diclofenac 75 mg/2 mL. Simultaneously with magnetic resonance imaging of the depot, a clinical-chemical evaluation and quantification of diclofenac in plasma was performed. A manifold enhancement of the T2-weighted magnetic resonance signal was observed in a muscle area of approximately 60 mL volume, with maximum signal intensity 30 min after injection, the time of maximum diclofenac plasma exposure. Plasma creatine kinase activity was elevated approximately sixfold within 8 h and normalized within 1 week, whereas the magnetic resonance enhancement disappeared within 5 weeks. Interestingly, the patient did not complain about any clinical symptoms at the injection site. Asymptomatic tissue injury after intramuscular injection of diclofenac, caused by intramuscular dosing, can be reliably evaluated by magnetic resonance imaging and should be applied early during the development of parenteral dosage forms. Clinical Trials Registration Number: BB130/16 (Ethics Committee of the University Medicine Greifswald).
Conflict of interest statement
Funding
This work was supported by the InnoProfile grant COM_DAT [03IPT612X] of the German Federal Ministry of Education and Research (BMBF).
Conflicts of interest
Mareike Probst, Jens-Peter Kühn, Christiane Modeß, Eberhard Scheuch, Anne Seidlitz, Norbert Hosten, Werner Siegmund and Werner Weitschies declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
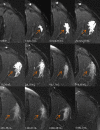
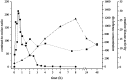
References
-
- Serratrice G. Study of diclofenac injectable. Tribune Med. 1982;46:43–48.
-
- Hegan T. Non steroidals, needles and negligence. Medical Protection Society casebook. Leeds: Medical Protection Society; 2002. pp. 12–13.
-
- O’Sullivan DP. Intramuscular diclofenac: 25 years worldwide safety perspective is vital to consider. Response to: Wright PJ, English PJ, Hungin APS, Marsden SNE. Managing acute renal colic across the primary-secondary care interface: a pathway of care based on evidence and consensus. BMJ. 2002;325:1408–1412. doi: 10.1136/bmj.325.7377.1408. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

