Clinical and Serologic Responses After a Two-dose Series of High-dose Influenza Vaccine in Plasma Cell Disorders: A Prospective, Single-arm Trial
- PMID: 28343904
- PMCID: PMC5413398
- DOI: 10.1016/j.clml.2017.02.025
Clinical and Serologic Responses After a Two-dose Series of High-dose Influenza Vaccine in Plasma Cell Disorders: A Prospective, Single-arm Trial
Abstract
Background: Patients with multiple myeloma (MM) and other plasma cell disorders are highly susceptible to influenza infections, which are major causes of morbidity in this population, despite the routine administration of a seasonal influenza vaccination. Existing data are limited by small and retrospective studies, which suggest poor seroprotection rates of < 20% after standard influenza vaccination in patients with MM.
Patients and methods: Patients with plasma cell dyscrasia (n = 51) were treated with a 2-dose series of high-dose inactivated trivalent influenza vaccine during the 2014 to 2015 influenza season. Laboratory-confirmed influenza infections were identified through seasonal surveillance, sera were collected for influenza hemagglutination antibody inhibition (HAI) titer assays, and logistic regression models were used to identify the clinical correlates to the HAI serologic responses.
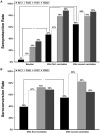
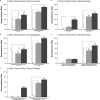
Results: Influenza vaccine was well tolerated, without any vaccine-related grade ≥ 2 adverse events. Only 3 patients (6%) experienced laboratory-confirmed influenza. The rates of HAI seroprotection against all 3 vaccine strains (A/California/7/2009 [H1N1] pdm09-like virus; A/Texas/50/2012 [H3N2]-like virus; and a B/Massachusetts/2/2012-like virus) increased from 4% at baseline to 49% and 65% after 1 and 2 doses, respectively. The risk factors associated with a lower likelihood of HAI serologic response included plasma cell disorder requiring therapy, less than a partial response found on disease response assessment, and active conventional chemotherapy. Alternatively, active therapy with an immunomodulatory drug alone or with a proteasome inhibitor was associated with a greater likelihood of an HAI serologic response.
Conclusion: These data have demonstrated that, in contrast to the historically poor results with standard influenza vaccination, this novel high-dose booster vaccination strategy leads to high rates of seroprotection. Randomized controlled studies are needed to compare this novel strategy to the standard vaccination strategy.
Keywords: Immunity; Infections; Monoclonal gammopathy of undetermined significance; Multiple myeloma; Vaccination.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Boccadoro M, Pileri A. Plasma cell dyscrasias: classification, clinical and laboratory characteristics, and differential diagnosis. Baillieres Clin Haematol. 1995;8(4):705–19. - PubMed
-
- Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211–25. - PubMed
-
- Centers for Disease C, Prevention. Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–62. - PubMed
-
- Mills KH, Cawley JC. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol. 1983;53(2):271–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

