Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes
- PMID: 28344001
- PMCID: PMC5390118
- DOI: 10.1016/j.stemcr.2017.02.016
Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes
Abstract
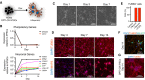
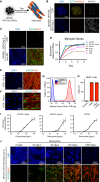
The isolation or in vitro derivation of many human cell types remains challenging and inefficient. Direct conversion of human pluripotent stem cells (hPSCs) by forced expression of transcription factors provides a potential alternative. However, deficient inducible gene expression in hPSCs has compromised efficiencies of forward programming approaches. We have systematically optimized inducible gene expression in hPSCs using a dual genomic safe harbor gene-targeting strategy. This approach provides a powerful platform for the generation of human cell types by forward programming. We report robust and deterministic reprogramming of hPSCs into neurons and functional skeletal myocytes. Finally, we present a forward programming strategy for rapid and highly efficient generation of human oligodendrocytes.
Keywords: human pluripotent stem cells; neurons; oligodendrocyte progenitor cells; reprogramming; skeletal myocytes.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Baron U., Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 2000;327:401–421. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

