Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells
- PMID: 28344002
- PMCID: PMC5390136
- DOI: 10.1016/j.stemcr.2017.02.021
Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells
Abstract
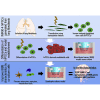
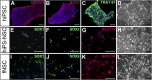
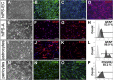
In vitro models of the human blood-brain barrier (BBB) are highly desirable for drug development. This study aims to analyze a set of ten different BBB culture models based on primary cells, human induced pluripotent stem cells (hiPSCs), and multipotent fetal neural stem cells (fNSCs). We systematically investigated the impact of astrocytes, pericytes, and NSCs on hiPSC-derived BBB endothelial cell function and gene expression. The quadruple culture models, based on these four cell types, achieved BBB characteristics including transendothelial electrical resistance (TEER) up to 2,500 Ω cm2 and distinct upregulation of typical BBB genes. A complex in vivo-like tight junction (TJ) network was detected by freeze-fracture and transmission electron microscopy. Treatment with claudin-specific TJ modulators caused TEER decrease, confirming the relevant role of claudin subtypes for paracellular tightness. Drug permeability tests with reference substances were performed and confirmed the suitability of the models for drug transport studies.
Keywords: blood-brain barrier (BBB) model; human induced pluripotent stem cells (hiPSCs); multipotent fetal neural stem cells (fNSCs); neurovascular unit in vitro.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Avdeef A., Deli M.A., Neuhaus W. John Wiley; 2015. In Vitro Assays for Assessing BBB Permeability. Blood-brain Barrier in Drug Discovery; pp. 188–237.
-
- Boyer-Di Ponio J., El-Ayoubi F., Glacial F., Ganeshamoorthy K., Driancourt C., Godet M., Perriere N., Guillevic O., Couraud P.O., Uzan G. Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS One. 2014;9:e84179. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

