Development of Peptidomimetic Inhibitors of the ERG Gene Fusion Product in Prostate Cancer
- PMID: 28344039
- PMCID: PMC5443258
- DOI: 10.1016/j.ccell.2017.02.017
Development of Peptidomimetic Inhibitors of the ERG Gene Fusion Product in Prostate Cancer
Erratum in
-
Development of Peptidomimetic Inhibitors of the ERG Gene Fusion Product in Prostate Cancer.Cancer Cell. 2017 Jun 12;31(6):844-847. doi: 10.1016/j.ccell.2017.05.001. Cancer Cell. 2017. PMID: 28609659 No abstract available.
-
Development of Peptidomimetic Inhibitors of the ERG Gene Fusion Product in Prostate Cancer.Cancer Cell. 2024 Aug 12;42(8):1471. doi: 10.1016/j.ccell.2024.07.009. Cancer Cell. 2024. PMID: 39137731 Free PMC article. No abstract available.
Abstract
Transcription factors play a key role in the development of diverse cancers, and therapeutically targeting them has remained a challenge. In prostate cancer, the gene encoding the transcription factor ERG is recurrently rearranged and plays a critical role in prostate oncogenesis. Here, we identified a series of peptides that interact specifically with the DNA binding domain of ERG. ERG inhibitory peptides (EIPs) and derived peptidomimetics bound ERG with high affinity and specificity, leading to proteolytic degradation of the ERG protein. The EIPs attenuated ERG-mediated transcription, chromatin recruitment, protein-protein interactions, cell invasion and proliferation, and tumor growth. Thus, peptidomimetic targeting of transcription factor fusion products may provide a promising therapeutic strategy for prostate cancer as well as other malignancies.
Keywords: ERG transcription factor; peptidomimetic inhibitor; prostate cancer.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
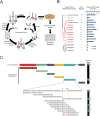
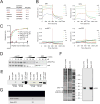
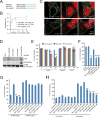

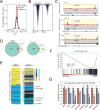
Comment in
-
Prostate cancer: Peptidomimetics have potential.Nat Rev Urol. 2017 Jun;14(6):328. doi: 10.1038/nrurol.2017.59. Epub 2017 Apr 11. Nat Rev Urol. 2017. PMID: 28401956 No abstract available.
References
-
- Albihn A, Johnsen JI, Henriksson MA. MYC in oncogenesis and as a target for cancer therapies. Adv Cancer Res. 2010;107:163–224. - PubMed
-
- An J, Ren S, Murphy SJ, Dalangood S, Chang C, Pang X, Cui Y, Wang L, Pan Y, Zhang X, et al. Truncated ERG Oncoproteins from TMPRSS2-ERG Fusions Are Resistant to SPOP-Mediated Proteasome Degradation. Molecular cell. 2015;59:904–916. - PubMed
-
- Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

