Effects of Food on the Pharmacokinetics of Omega-3-Carboxylic Acids in Healthy Japanese Male Subjects: A Phase I, Randomized, Open-label, Three-period, Crossover Trial
- PMID: 28344197
- PMCID: PMC5587524
- DOI: 10.5551/jat.38737
Effects of Food on the Pharmacokinetics of Omega-3-Carboxylic Acids in Healthy Japanese Male Subjects: A Phase I, Randomized, Open-label, Three-period, Crossover Trial
Abstract
Aims: Omega-3-carboxylic acids (OM3-CA) contain omega-3 free fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as carboxylic acids. Food intake is known to affect the bioavailability of ethyl ester fatty acid formulations. We conducted a phase I study to investigate the effects of the timing of OM3-CA administration relative to food intake on the pharmacokinetics of EPA and DHA.
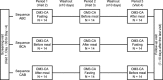
Methods: In this randomized, open-label, three-period crossover study, Japanese healthy male subjects were administered 4×1 g OM3-CA capsules with continued fasting, before a meal, or after a meal. All subjects fasted for ≥10 h prior to drug/meal administration. The primary objective was to examine the effect of meal timing on the pharmacokinetics of EPA and DHA after OM3-CA administration. The secondary objectives were to examine the safety and tolerability of OM3-CA.
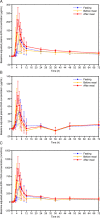
Results: A total of 42 Japanese subjects was enrolled in the study. The baseline-adjusted maximum concentration and area under the concentration-time curve from 0 to 72 h for EPA, DHA, and EPA +DHA were lower in the fasting and before meal conditions than in the after meal condition. The maximum total EPA, total DHA, and total EPA+DHA concentrations were reached later when administered in fasting conditions than in fed conditions, indicating slower absorption in fasting conditions. Diarrhea was reported by five, six, and no subjects in the fasting, before meal, and after meal conditions, respectively.
Conclusions: The timing of OM3-CA administration relative to food intake influences the systemic bioavailability of EPA and DHA in healthy Japanese male subjects.
Trial registration: NCT02372344.
Keywords: Docosahexaenoic acid; Eicosapentaenoic acid; Japan; Omega-3 fatty acids; Pharmacokinetics.
Conflict of interest statement
Y. Noda, H. Shimada, H. Kim and T. Yajima are employees of AstraZeneca K.K., Japan. C. Nilsson and T. Lundström are employees of AstraZeneca Gothenburg, Mölndal, Sweden.
Figures
References
-
- Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 - PubMed
-
- Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y, JELIS Investigators : Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J, 2009; 73: 1283-1290 - PubMed
-
- Nakamura N, Hamazaki T, Kobayashi M, Ohta M, Okuda K: Effects of eicosapentaenoic acids on remnant-like particles, cholesterol concentrations and plasma fatty acid composition in patients with diabetes mellitus. In Vivo, 1998; 12: 311-314 - PubMed
-
- Nomura S, Kanazawa S, Fukuhara S: Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with type 2 diabetes mellitus. J Diabetes Complications, 2003; 17: 153-159 - PubMed
-
- Okumura T, Fujioka Y, Morimoto S, Tsuboi S, Masai M, Tsujino T, Ohyanagi M, Iwasaki T: Eicosapentaenoic acid improves endothelial function in hypertriglyceridemic subjects despite increased lipid oxidizability. Am J Med Sci, 2002; 324: 247-253 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

