High-Temperature Synthesis of Ordered Mesoporous Aluminosilicates from ZSM-5 Nanoseeds with Improved Acidic Properties
- PMID: 28344243
- PMCID: PMC5304696
- DOI: 10.3390/nano4030712
High-Temperature Synthesis of Ordered Mesoporous Aluminosilicates from ZSM-5 Nanoseeds with Improved Acidic Properties
Abstract
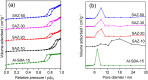
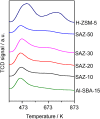
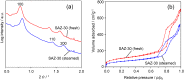
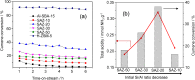
Ordered mesoporous SBA-15 analogs with different Si/Al ratios were successfully prepared in a two-step process from self-assembly of ZSM-5 nanoseeds at high temperature in mildly acidic media (473 K, pH 3.5). The obtained products were characterized as SAXS, XRD, N₂ sorption, FTIR, TEM, NH₃-TPD, AAS and ICP. The results show that the initial Si/Al molar ratio of ZSM-5 precursors strongly affects the final materials' properties. A highly condensed, well-ordered mesoporous SBA-15 analog with improved hydrothermal stability and acidic properties can be prepared from low aluminum containing ZSM-5 precursors (Si/Al ≥ 20). Reducing the initial Si/Al molar ratio to 10, however, leads to the formation of a disordered mesoporous SBA-15 type material accompanied by degraded textural and acidic properties. The gas phase cracking of cumene, carried out as probe reaction to evaluate Brønsted acidity, reveals that an increased density of Brønsted acid sites has been achieved over the SBA-15 analogs compared to conventional Al-SBA-15 due to the preservation of zeolite building units in the mesopore walls of the SBA-15 analogs.
Keywords: SBA-15 analogs; acidity; cumene cracking; hydrothermal stability; zeolite nanoseeds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Corma A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003;216:298–312. doi: 10.1016/S0021-9517(02)00132-X. - DOI
-
- Stöcker M. Gas phase catalysis by zeolites. Microporous Mesoporous Mater. 2005;82:257–292. doi: 10.1016/j.micromeso.2005.01.039. - DOI
-
- Čejka J., Mintova S. Perspectives of micro/mesoporous composites in catalysis. Catal. Rev. Sci. Eng. 2007;49:457–509. doi: 10.1080/01614940701583240. - DOI
-
- Liu Y., Pinnavaia T.J. Aluminosilicate mesostructures with improved acidity and hydrothermal stability. J. Mater. Chem. 2002;12:3179–3190. doi: 10.1039/b204094h. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

