Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability
- PMID: 28344251
- PMCID: PMC5304692
- DOI: 10.3390/nano4030827
Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability
Abstract
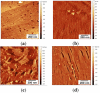
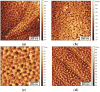
Hydrophobins are small proteins secreted by fungi and which spontaneously assemble into amphipathic layers at hydrophilic-hydrophobic interfaces. We have examined the self-assembly of the Class I hydrophobins EAS∆15 and DewA, the Class II hydrophobin NC2 and an engineered chimeric hydrophobin. These Class I hydrophobins form layers composed of laterally associated fibrils with an underlying amyloid structure. These two Class I hydrophobins, despite showing significant conformational differences in solution, self-assemble to form fibrillar layers with very similar structures and require a hydrophilic-hydrophobic interface to trigger self-assembly. Addition of additives that influence surface tension can be used to manipulate the fine structure of the protein films. The Class II hydrophobin NC2 forms a mesh-like protein network and the engineered chimeric hydrophobin displays two multimeric forms, depending on assembly conditions. When formed on a graphite surface, the fibrillar EAS∆15 layers are resistant to alcohol, acid and basic washes. In contrast, the NC2 Class II monolayers are dissociated by alcohol treatment but are relatively stable towards acid and base washes. The engineered chimeric Class I/II hydrophobin shows increased stability towards alcohol and acid and base washes. Self-assembled hydrophobin films may have extensive applications in biotechnology where biocompatible; amphipathic coatings facilitate the functionalization of nanomaterials.
Keywords: amphipathic; film; hydrophobin; protein; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Solution structure and interface-driven self-assembly of NC2, a new member of the Class II hydrophobin proteins.Proteins. 2014 Jun;82(6):990-1003. doi: 10.1002/prot.24473. Epub 2013 Dec 26. Proteins. 2014. PMID: 24218020
-
Predicting the self-assembly film structure of class II hydrophobin NC2 and estimating its structural characteristics.Colloids Surf B Biointerfaces. 2020 Nov;195:111269. doi: 10.1016/j.colsurfb.2020.111269. Epub 2020 Jul 26. Colloids Surf B Biointerfaces. 2020. PMID: 32739772
-
Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials.Adv Exp Med Biol. 2019;1174:161-185. doi: 10.1007/978-981-13-9791-2_5. Adv Exp Med Biol. 2019. PMID: 31713199 Review.
-
Formation of Amphipathic Amyloid Monolayers from Fungal Hydrophobin Proteins.Methods Mol Biol. 2020;2073:55-72. doi: 10.1007/978-1-4939-9869-2_4. Methods Mol Biol. 2020. PMID: 31612436
-
Hydrophobins: multipurpose proteins.Annu Rev Microbiol. 2001;55:625-46. doi: 10.1146/annurev.micro.55.1.625. Annu Rev Microbiol. 2001. PMID: 11544369 Review.
Cited by
-
Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins.Biomolecules. 2017 Jun 26;7(3):45. doi: 10.3390/biom7030045. Biomolecules. 2017. PMID: 28672843 Free PMC article. Review.
-
A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne, Australia.PLoS One. 2020 Dec 18;15(12):e0238901. doi: 10.1371/journal.pone.0238901. eCollection 2020. PLoS One. 2020. PMID: 33338037 Free PMC article.
-
Hydrophobins: multifunctional biosurfactants for interface engineering.J Biol Eng. 2019 Jan 23;13:10. doi: 10.1186/s13036-018-0136-1. eCollection 2019. J Biol Eng. 2019. PMID: 30679947 Free PMC article. Review.
-
Surface Functionalization by Hydrophobin-EPSPS Fusion Protein Allows for the Fast and Simple Detection of Glyphosate.Biosensors (Basel). 2019 Aug 29;9(3):104. doi: 10.3390/bios9030104. Biosensors (Basel). 2019. PMID: 31470576 Free PMC article.
-
The good, the bad and the tasty: The many roles of mushrooms.Stud Mycol. 2016 Sep;85:125-157. doi: 10.1016/j.simyco.2016.11.002. Epub 2016 Nov 11. Stud Mycol. 2016. PMID: 28082758 Free PMC article.
References
-
- Zykwinska A., Pihet M., Radji S., Bouchara J.P., Cuenot S. Self-assembly of proteins into a three-dimensional multilayer system: Investigation of the surface of the human fungal pathogen aspergillus fumigatus. Biochim. Biophys. Acta Proteins Proteomics. 2014;1844:1137–1144. doi: 10.1016/j.bbapap.2014.03.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

