Impact of the De-Alloying Kinetics and Alloy Microstructure on the Final Morphology of De-Alloyed Meso-Porous Metal Films
- PMID: 28344253
- PMCID: PMC5308459
- DOI: 10.3390/nano4040856
Impact of the De-Alloying Kinetics and Alloy Microstructure on the Final Morphology of De-Alloyed Meso-Porous Metal Films
Abstract
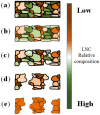
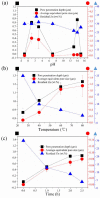
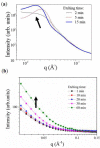
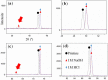
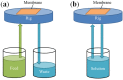
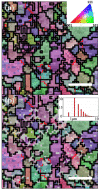
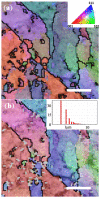
Nano-textured porous metal materials present unique surface properties due to their enhanced surface energy with potential applications in sensing, molecular separation and catalysis. In this paper, commercial alloy foils, including brass (Cu85Zn15 and Cu70Zn30) and white gold (Au50Ag50) foils have been chemically de-alloyed to form nano-porous thin films. The impact of the initial alloy micro-structure and number of phases, as well as chemical de-alloying (DA) parameters, including etchant concentration, time and solution temperature on the final nano-porous thin film morphology and properties were investigated by electron microscopy (EM). Furthermore, the penetration depth of the pores across the alloys were evaluated through the preparation of cross sections by focus ion beam (FIB) milling. It is demonstrated that ordered pores ranging between 100 nm and 600 nm in diameter and 2-5 μm in depth can be successfully formed for the range of materials tested. The microstructure of the foils were obtained by electron back-scattered diffraction (EBSD) and linked to development of pits across the material thickness and surface during DA. The role of selective etching of both noble and sacrificial metal phases of the alloy were discussed in light of the competitive surface etching across the range of microstructures and materials tested.
Keywords: de-alloying (DA) kinetics; metal surface texture; micro-structure morphology relationship; through pores formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dumée L.F., He L., Lin B., Ailloux F.-M., Lemoine J.-B., Velleman L., She F., Duke M.C., Orbell J.D., Erskine G., et al. The fabrication and surface functionalization of porous metal frameworks—A review. J. Mater. Chem. A. 2013;1:15185–15206. doi: 10.1039/c3ta13240d. - DOI
-
- Kramer D., Viswanath R.N., Weissmüller J. Surface-stress induced macroscopic bending of nanoporous gold cantilevers. Nano Lett. 2004;4:793–796. doi: 10.1021/nl049927d. - DOI
-
- Newman R., Mehta A. An AC impedance study of the de-alloying of Fe-Ni alloys, and its relevance to chloride SCC of stainless steels. Corros. Sci. 1988;28:1183–1187. doi: 10.1016/0010-938X(88)90127-8. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

