Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles
- PMID: 28344259
- PMCID: PMC5302549
- DOI: 10.3390/nano6010002
Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles
Abstract
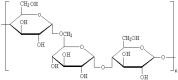
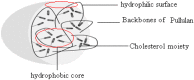
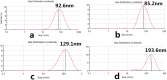
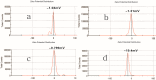
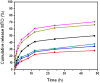
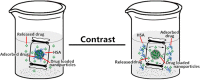
We prepared two types of cholesterol hydrophobically modified pullulan nanoparticles (CHP) and carboxyethyl hydrophobically modified pullulan nanoparticles (CHCP) substituted with various degrees of cholesterol, including 3.11, 6.03, 6.91 and 3.46 per polymer, and named CHP-3.11, CHP-6.03, CHP-6.91 and CHCP-3.46. Dynamic laser light scattering (DLS) showed that the pullulan nanoparticles were 80-120 nm depending on the degree of cholesterol substitution. The mean size of CHCP nanoparticles was about 160 nm, with zeta potential -19.9 mV, larger than CHP because of the carboxyethyl group. A greater degree of cholesterol substitution conferred greater nanoparticle hydrophobicity. Drug-loading efficiency depended on nanoparticle hydrophobicity, that is, nanoparticles with the greatest degree of cholesterol substitution (6.91) showed the most drug encapsulation efficiency (90.2%). The amount of drug loading increased and that of drug release decreased with enhanced nanoparticle hydrophobicity. Nanoparticle surface-negative charge disturbed the amount of drug loading and drug release, for an opposite effect relative to nanoparticle hydrophobicity. The drug release in pullulan nanoparticles was higher pH 4.0 than pH 6.8 media. However, the changed drug release amount was not larger for negative-surface nanoparticles than CHP nanoparticles in the acid release media. Drug release of pullulan nanoparticles was further slowed with human serum albumin complexation and was little affected by nanoparticle hydrophobicity and surface negative charge.
Keywords: HSA; degree of substitution; pullulan nanoparticles; surface charge.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources

