New knowledge of the mechanisms of sorafenib resistance in liver cancer
- PMID: 28344323
- PMCID: PMC5457690
- DOI: 10.1038/aps.2017.5
New knowledge of the mechanisms of sorafenib resistance in liver cancer
Abstract
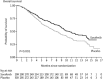
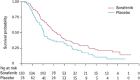
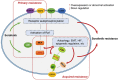
Sorafenib is an oral multikinase inhibitor that suppresses tumor cell proliferation and angiogenesis and promotes tumor cell apoptosis. It was approved by the FDA for the treatment of advanced renal cell carcinoma in 2006, and as a unique target drug for advanced hepatocellular carcinoma (HCC) in 2007. Sorafenib can significantly extend the median survival time of patients but only by 3-5 months. Moreover, it is associated with serious adverse side effects, and drug resistance often develops. Therefore, it is of great importance to explore the mechanisms underlying sorafenib resistance and to develop individualized therapeutic strategies for coping with these problems. Recent studies have revealed that in addition to the primary resistance, several mechanisms are underlying the acquired resistance to sorafenib, such as crosstalk involving PI3K/Akt and JAK-STAT pathways, the activation of hypoxia-inducible pathways, and epithelial-mesenchymal transition. Here, we briefly describe the function of sorafenib, its clinical application, and the molecular mechanisms for drug resistance, especially for HCC patients.
Figures

References
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. - PubMed
-
- Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, et al. Phase II trial of single-agent sorafenib in patients with advanced non-small-cell lung carcinoma. J Clin Oncol 2006; 24: 364. - PubMed
-
- Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer 2007; 97: 1480–5. - PMC - PubMed
-
- Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM Jr, Wiesenfeld M, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol 2009; 27: 11–5. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

