Metal homeostasis and resistance in bacteria
- PMID: 28344348
- PMCID: PMC5963929
- DOI: 10.1038/nrmicro.2017.15
Metal homeostasis and resistance in bacteria
Erratum in
-
Corrigendum: Metal homeostasis and resistance in bacteria.Nat Rev Microbiol. 2017 May 12;15(6):379. doi: 10.1038/nrmicro.2017.53. Nat Rev Microbiol. 2017. PMID: 28496160 No abstract available.
Abstract
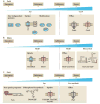
Metal ions are essential for many reactions, but excess metals can be toxic. In bacteria, metal limitation activates pathways that are involved in the import and mobilization of metals, whereas excess metals induce efflux and storage. In this Review, we highlight recent insights into metal homeostasis, including protein-based and RNA-based sensors that interact directly with metals or metal-containing cofactors. The resulting transcriptional response to metal stress takes place in a stepwise manner and is reinforced by post-transcriptional regulatory systems. Metal limitation and intoxication by the host are evolutionarily ancient strategies for limiting bacterial growth. The details of the resulting growth restriction are beginning to be understood and seem to be organism-specific.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

