Ferroelectric switching and electrochemistry of pyrrole substituted trialkylbenzene-1,3,5-tricarboxamides
- PMID: 28344384
- PMCID: PMC5347932
- DOI: 10.1002/polb.24318
Ferroelectric switching and electrochemistry of pyrrole substituted trialkylbenzene-1,3,5-tricarboxamides
Abstract
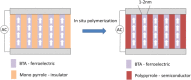
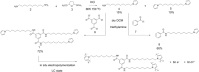
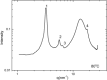
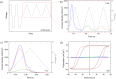
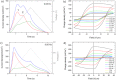
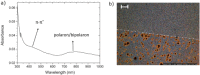
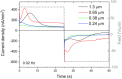
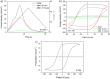
We explore a new approach to organic ferroelectric diodes using a benzene-tricarboxamide (BTA) core connected with C10 alkyl chains to pyrrole groups, which can be polymerized to provide a semiconducting ferroelectric material. The compound possesses a columnar hexagonal liquid crystalline (LC) phase and exhibits ferroelectric switching. At low switching frequencies, an additional process occurs, which leads to a high hysteretic charge density of up to ∼1000 mC/m2. Based on its slow rate, the formation of gas bubbles, and the emergence of characteristic polypyrrole absorption bands in the UV-Vis-NIR, the additional process is identified as the oxidative polymerization of pyrrole groups, enabled by the presence of amide groups. Polymerization of the pyrrole groups, which is essential to obtain semiconductivity, is limited to thin layers at the electrodes, amounting to ∼17 nm after cycling for 21 h. © 2017 Wiley Periodicals, Inc. J. Polym. Sci., Part B: Polym. Phys. 2017, 55, 673-683.
Keywords: electropolymerization; ferroelectric liquid crystal; interface; nanomaterials; polypyrrole.
Figures


References
-
- Horiuchi S., Tokura Y., Nat. Mater. 2008, 7, 357–366. - PubMed
-
- Naber R. C. G., Asadi K., Blom P. W. M., de Leeuw D. M., de Boer B., Adv. Mater. 2010, 22, 933–945. - PubMed
-
- Asadi K., de Leeuw D. M., de Boer B., Blom P. W. M., Nat. Mater. 2008, 7, 547–550. - PubMed
-
- Kemerink M., Asadi K., Blom P. W. M., de Leeuw D. M., Org. Electron. 2012, 13, 147–152.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
